Solutions

Managing impact on Global Pharma and MedTech MNCs in the context of CBDT regulations
Ensuring CBDT compliance
With the introduction of CBDT regulation in China, there are major impacts foreseen with regards to MNCs business operations as well as any activity that involves data collection and transfer out of the country. We discuss actions MNCs can take to ensure CBDT compliance.
With the evolving regulatory landscape and the introduction of CBDT regulation in China, Deloitte foresees that it will create a significant impact on the MNC sector and other industries that are heavily reliant on data, one of which is healthcare and life sciences. The new measures will bring about changes to MNCs’ daily operations on various aspects to enhance data and CBDT security. It is critical for MNCs, especially those with operations in China, to prepare to mitigate or remediate the possible impacts of the new measures.
To strategically cope with and respond to CBDT regulations, enterprises should begin by identifying the involved data types and their respective volumes. Both mitigation and remediation strategies should also be considered. Mitigation strategy retains CBDT activities with policy and technical enhancement to ensure data security, application/tool security, and end-user security, while the remediation strategy discontinues CBDT activities through data localisation, data team localisation, and support team localisation. In the near future, organisations may potentially have to undergo a series of strategic and operational changes to cope with the new measures, thus, Deloitte has come up with a point of view in 5 crucial areas to manage China's CBDT compliance.
Managing impact on Global Pharma and MedTech MNCs in the context of China Cross-Border Data Transfer (CBDT) regulations
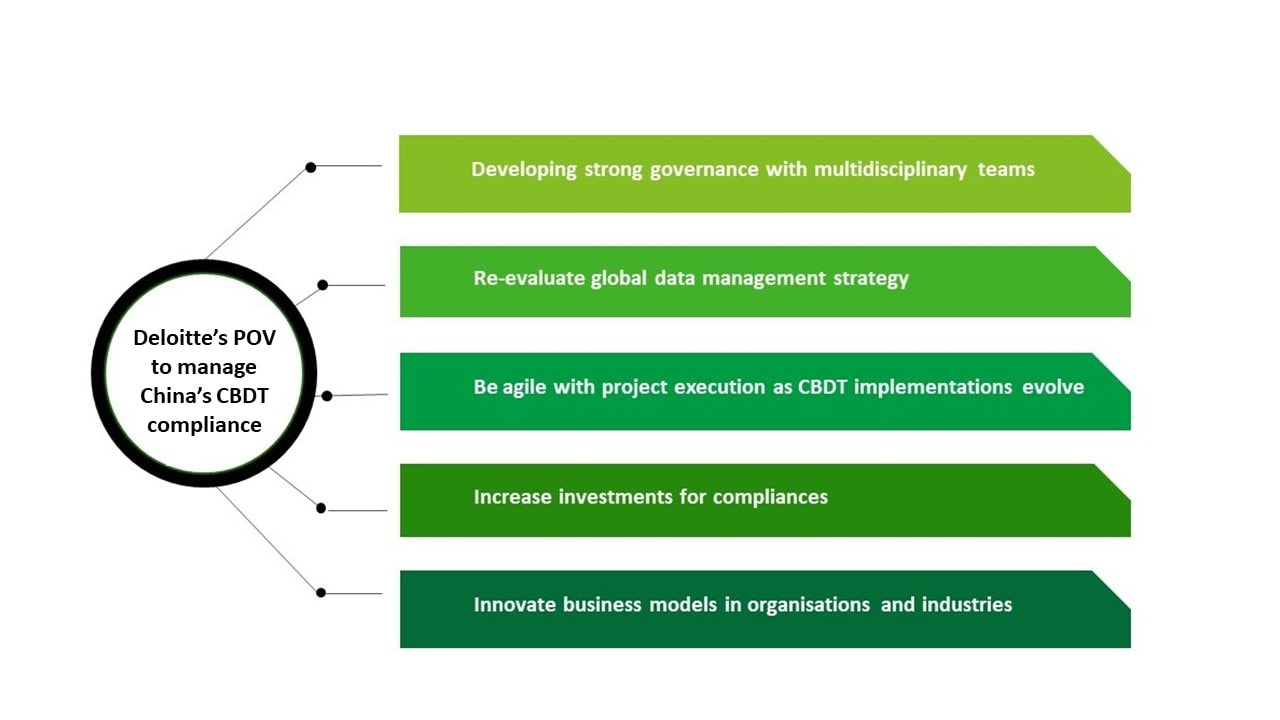
Download the paper to read more on how to navigate the impacts of China's new CBDT regulations and Deloitte's POV to manage China's CBDT compliances.
This report is based on CBDT status as of 31st March 2023.
Contact us
 |
Ashish Mahajan Technology & Transformation Executive Director Technology and Digital Transformation, Life Science & Healthcare Deloitte Southeast Asia asmahajan@deloitte.com |
 |
Steven Ye Feng Partner Cyber & Strategic Risk Deloitte China stefeng@deloitte.com.cn |
 |
Ilkka Matti Salminen Technology & Transformation Director Life Sciences & Health Care Deloitte Southeast Asia isalminen@deloitte.com |
 |
Dr. Manjie Xing Senior Consultant Life Sciences & Health Care Deloitte Southeast Asia mxing@deloitte.com |
Recommendations
Realising the promise of cell and gene therapies in Asia Pacific
Four imperatives for commercial delivery
The future of health
How innovation will blur traditional health care boundaries