Article

Identification of Medicinal Products
Connecting the parts
Identification of Medicinal Products (IDMP) is one the biggest regulatory challenges for all pharmaceutical companies operating in Europe. How can companies navigate this journey towards increased patient safety and use it as an opportunity for business transformation?
Explore Content
- Developing an integrated and holistic IDMP strategy
- Further reading
- Contacts
- Follow us on social media
What is IDMP?
IDMP regulation aims to uniquely identify pharmaceutical products, standardize product information and aid the exchange of information between pharmaceutical companies and global regulators all with the ultimate goal of improving patient safety.
Under the IDMP standards, all pharmaceutical and biotechnology companies will be required to electronically submit detailed product data for all of their products marketed in Europe and maintain it on an ongoing basis.
Five standards of IDMP:
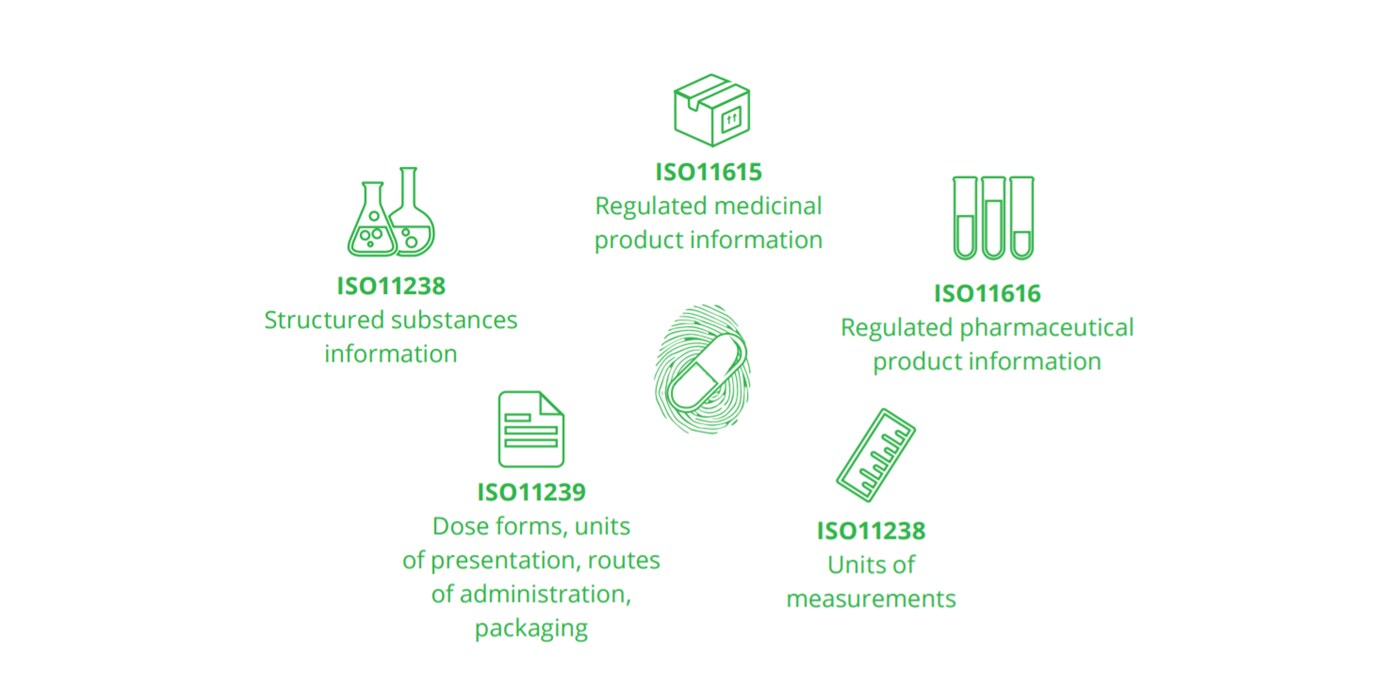
What needs to happen and when?
The roll out of IDMP will take an iterative approach, providing the industry with time to comply with the regulatory mandate.
The four iterations for data submission:
- Minimum required elements to assign identifiers for marketed products
- Full investigational product scope
- Remaining requirements for clinical particulars
- Remaining requirements for packaging and manufacturing
The timeline for IDMP however has been dynamic. While guidelines are expected to be released in 2018, the delay and changes in timing is providing life sciences companies with a window of opportunity to re-evaluate their strategic plans for IDMP, considering the synergies that exist with other regulatory mandates as well as the opportunities for cross functional collaboration and operational efficiency.
IDMP synergies with other regulations
IDMP shares a range of commonalities with other regulatory mandates, and will form the basis as a data standard for many of these future regulations. Considering IDMP as a part of a much larger regulatory landscape of initiatives will allow life sciences companies to leverage commonalities and synergies.
For more information on the synergies of IDMP with other regulations, view or download the full report: Connecting the parts: Developing an integrated IDMP strategy.
Beyond IDMP compliance
IDMP will lay the foundation for building an insight-driven organisation by delivering a standardised cross-functional data model based on regulated product master data and preparing the industry for future excellence.
Companies that go beyond the minimum requirements of IDMP can expect greater operational excellence and efficiency through improved data quality, increased collaboration and integration of technologies.
Developing an integrated and holistic IDMP strategy
A change of this scale generally leads to change in business operating models and IT transformations highlighting the need for an end to end, integrated change process involving these crucial steps:
- Understand the regulatory requirements and create awareness
- Understand the impact on business processes and identify any gaps or pain points
- Identify the remediation plan and actions
- Identify and create a strategic vision for implementing the change
- Analyze if people/data/system/processes need to be improved
- Present the business case
- Define implementation strategy based on the vision
Further reading:
Recommendations
The bigger picture
Impact of EU regulatory change on the global life sciences industry
Under the spotlight
Data integrity in life sciences

Follow us @DeloitteHealth