Optimizing market access How therapeutic area dynamics could influence strategy
19 minute read
14 March 2019
With public pressure to reduce drug spending, payers are increasingly using formulary tools to help manage market access. This article examines factors driving the success of market access strategies.
Executive summary
Market access strategy is complex, dynamic, and constantly evolving. Biopharma leaders can optimize market access across a portfolio of products by analyzing how therapeutic area (TA) dynamics, including the level of competition, could influence the use of payer tools to manage access. Over the last several years, payers have increasingly used formulary tiering as one tool to help control and manage market access. With intensifying public pressure to reduce drug spending, this trend is likely to continue. However, not all TAs are impacted equally. Our analysis of the degree to which competition drives formulary positioning across eight TAs reveals insights on what additional dynamics could be driving formulary positioning, a proxy for the success of market access strategies.
Learn more
A new view on market access and reimbursement in China
Read more from the Life sciences collection
Subscribe to receive related content from Deloitte Insights
Deloitte analyzed the branded portfolios of the largest 19 biopharma companies (by revenue) and the level of competition (branded and generic, direct and indirect) and the formulary tier position for each product. Interestingly, we found that formulary positioning appears to be driven by competition in some TAs (metabolic, cardiovascular, central nervous system, and gastrointestinal), but not others (oncology, infectious disease, immunology, and respiratory).
Comparing metabolic with oncology provides some insight into why competition might drive formulary positioning in some TAs and not in others. Drugs in the metabolic TA address diseases that impact a large proportion of the population, are effective, and offer limited clinical differentiation within classes. On the other hand, drugs in the oncology TA treat diseases with high unmet need, are expensive, and have varying levels of efficacy in different tumor types. Currently, payers may be less willing to limit market access for products that could address more immediate life-threating conditions. However, as newer and more efficacious treatments become available, and competition increases, we expect to see more efforts to control and manage access to oncology drugs as well.
Some of these dynamics could change as a result of ongoing discussions about reducing the influence of rebates on market access at both federal policymaking and private sector levels. Some private payers are already establishing strategies to reduce the influence of rebates on market access by passing on rebates at the point of sale or creating new formulary types, but it is yet to be seen how this could impact pricing and market access.
Favorable formulary positioning alone does not always dictate ultimate product performance and profitability. The cost and benefits of market access strategies such as rebating, value-based contracting, or patient services should be carefully considered. In addition, several tools can be leveraged to overcome payer pharmacy benefit designs and less favorable tiering. Market access leaders should develop sophisticated decision-making and analytics capabilities to track market dynamics as they evolve to help build the business case for investing in specific market access strategies.
Understanding market dynamics
One way to gauge the success—or failure—of a product’s market access strategy is to look at where it sits on formularies. Placement on lower co-pay tiers often suggests a successful strategy, while being on a high tier, with greater cost-sharing requirements of patients, can mean lower utilization and revenue, though not always. The profitability of strategies to obtain preferential formulary positioning should be considered. For this analysis, formulary positioning (and assumed associated co-pay amounts or co-insurance levels) is used as a proxy to measure the success of market access strategies.
Methodology
We scored 286 products according to their competitive intensity (branded, generic, direct, and indirect competitors) and formulary positioning (weighted score for percentage of covered populations under each tier level, or not covered). The formulary data was current as of September 2018. The higher the competitive intensity score (x-axis in the figures throughout this article), the more competitive pressure the product faces. The higher the formulary score (y-axis), the greater the percentage of the population covered under higher formulary tiers and/or do not have coverage.
We mapped these against one another to understand how competitive factors drive formulary positioning. Drugs that fall in the upper-right quadrant face the highest competitive intensity and have the highest formulary positions, while those that fall in lower-left quadrant have lower competitive intensity and lower formulary positioning.
We did this separately for commercial and Medicare populations.
Competition through direct (in-class) or indirect (outside of class) generic or branded competitors can impact formulary positioning. A drug with little competition in the market and strong clinical evidence may be able to secure better positioning on a formulary because the manufacturer has leverage. In contrast, a branded drug with multiple competitors, especially ones for which there is greater evidence around efficacy, may need to apply different tools to enhance market access.
We analyzed the relationship between competitive intensity and formulary positioning by therapeutic area, focusing on the top-selling branded drugs of the top 19 biopharma companies by their revenue for 2016. (See more details on the methodology in the “Methodology” sidebar and in Appendix I.)
As competitive intensity for metabolic drugs rises, so does formulary positioning
This is not the case, however, for oncology drugs
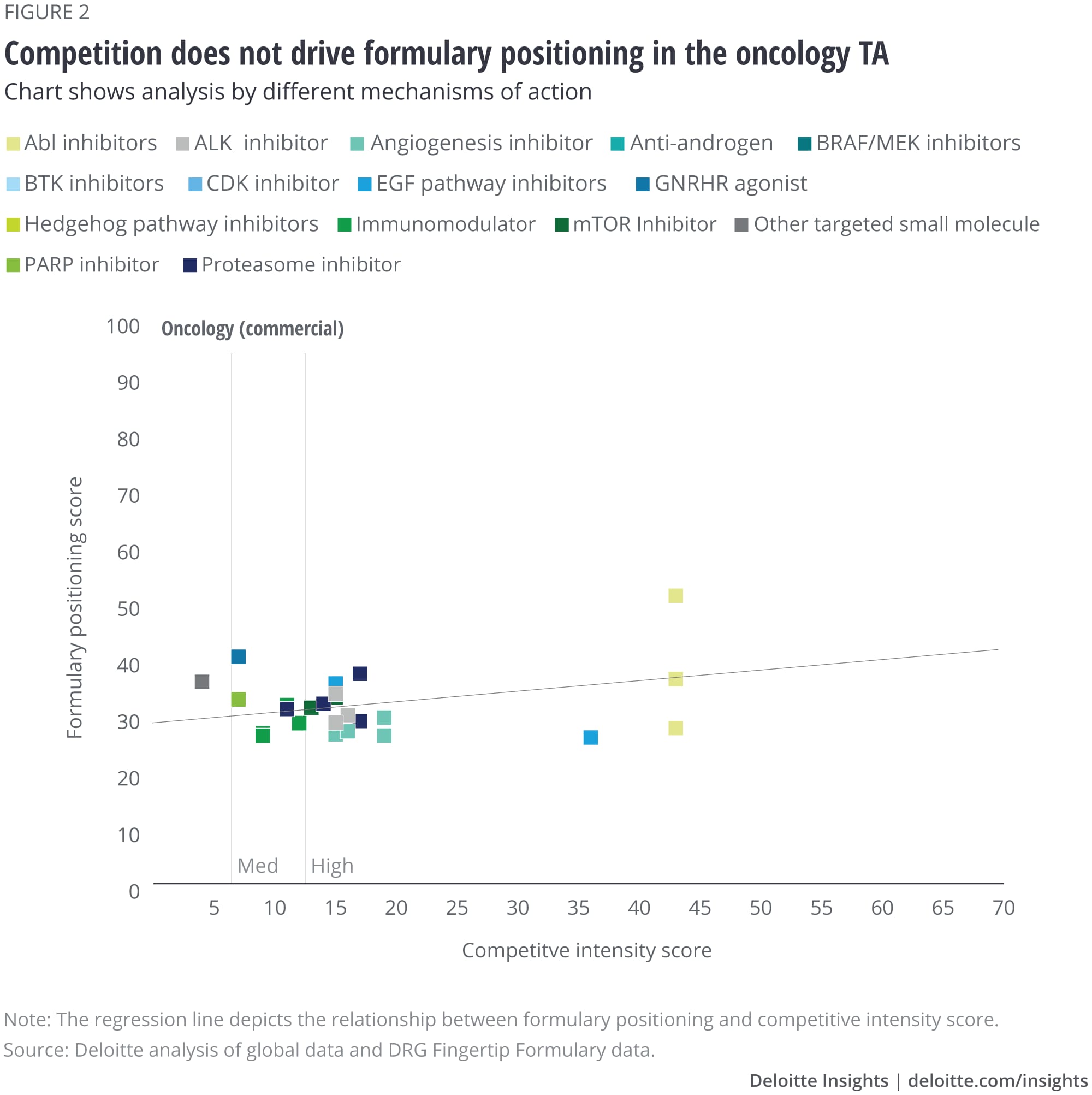
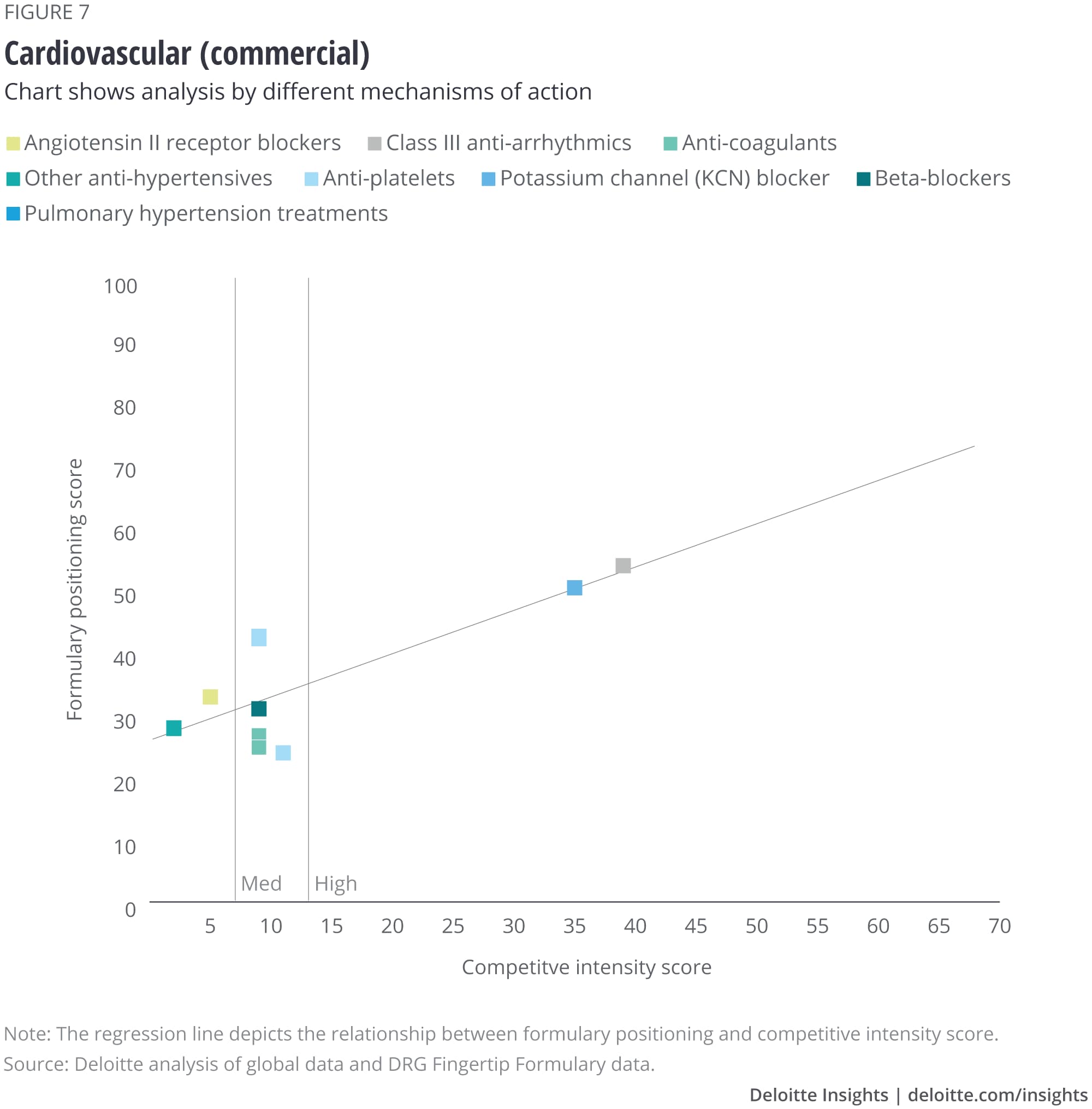
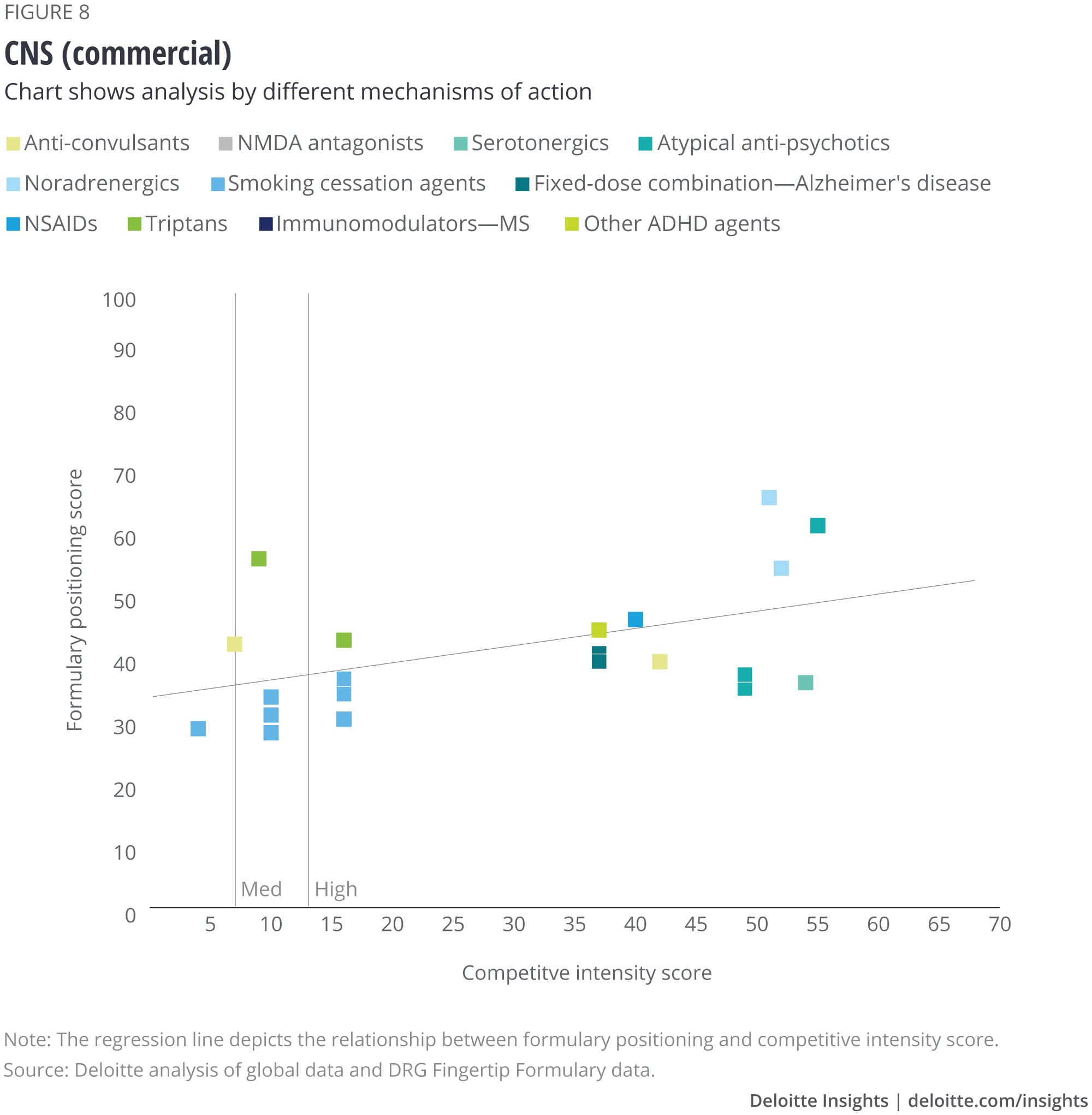
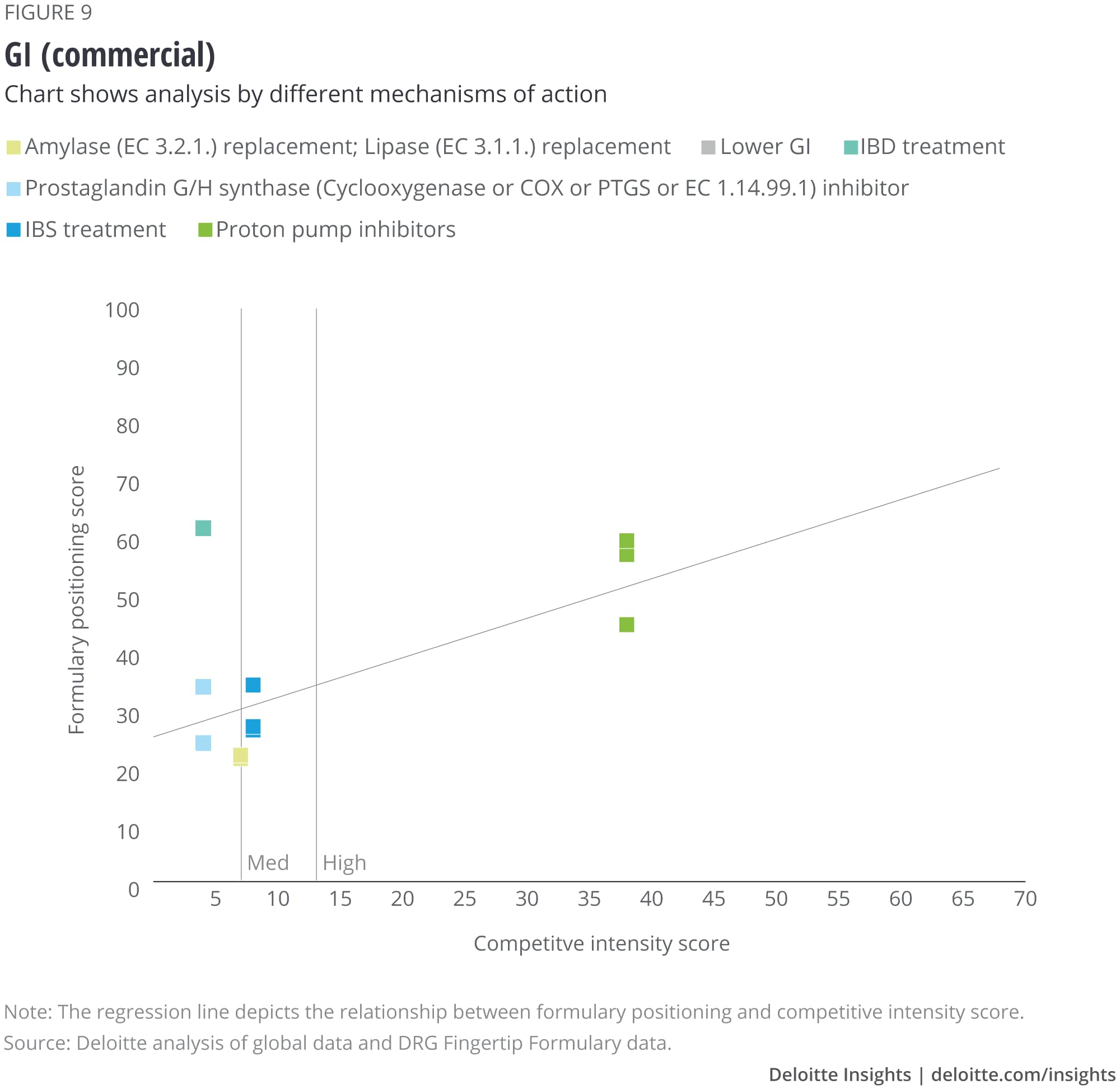
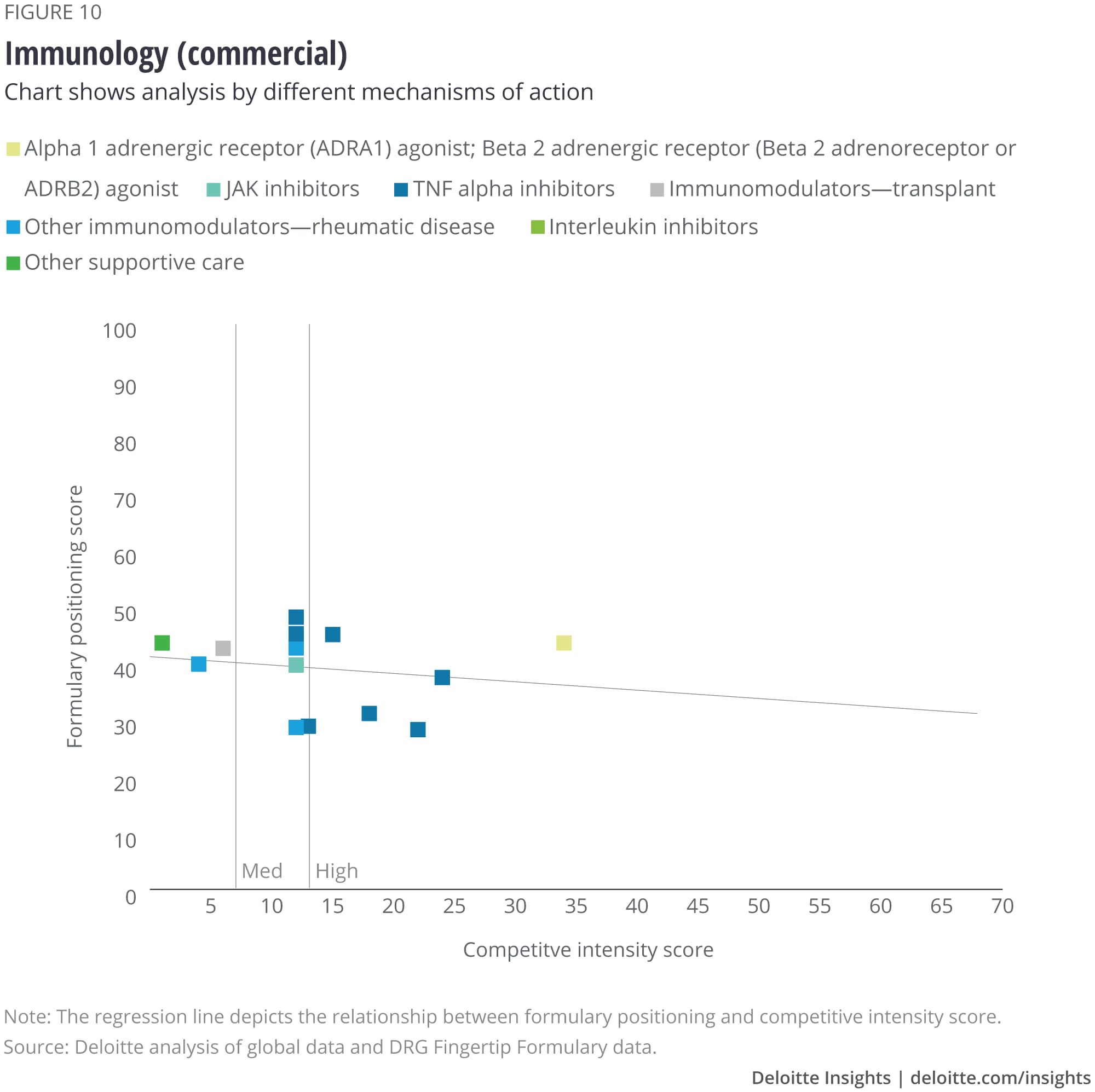
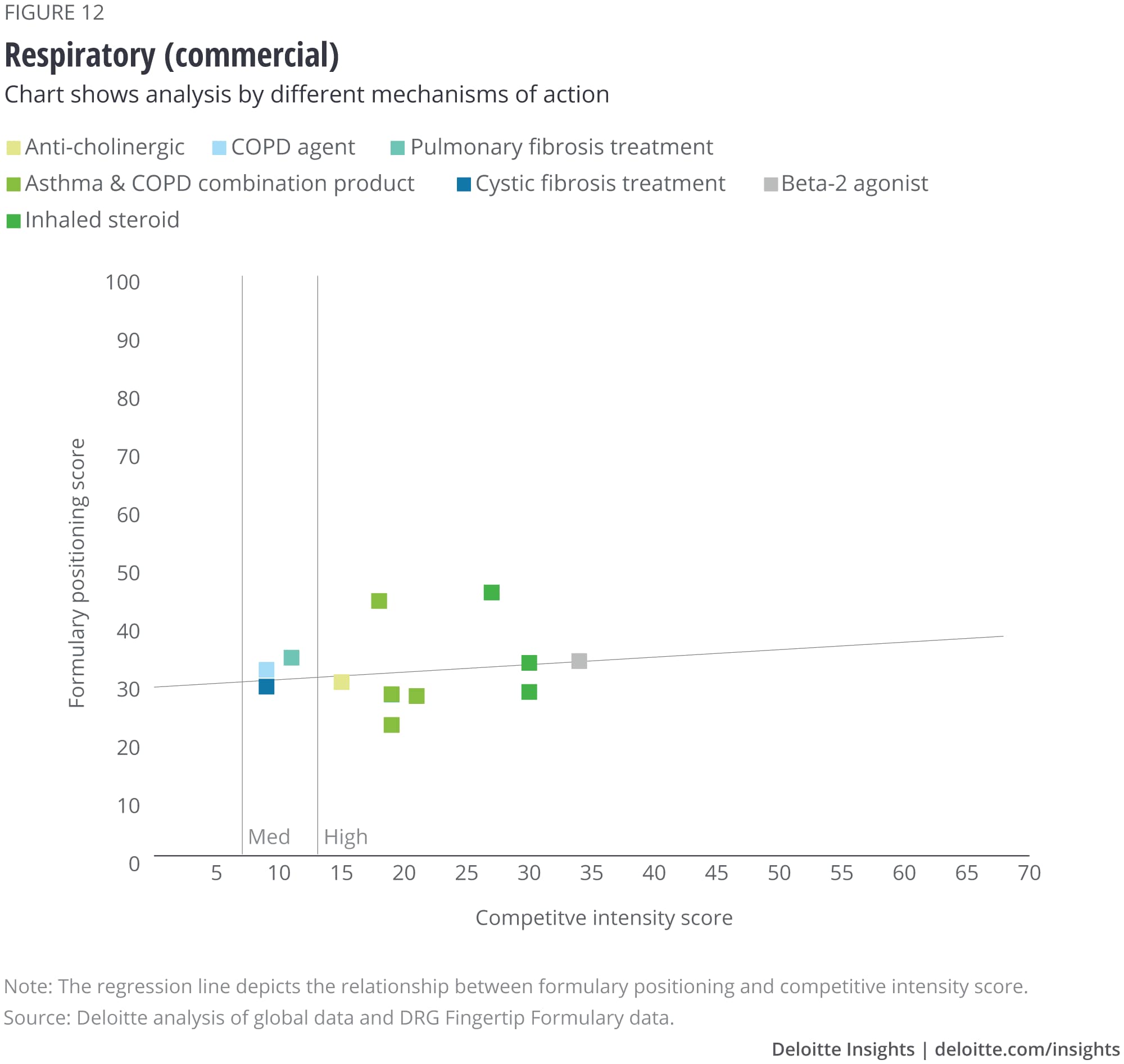
We analyzed the eight largest TAs among our set of drugs, and after removing outliers, we saw that some TAs show a correlation with formulary positioning in commercial plans, while others do not. The TAs that show a correlation are metabolic (figure 1), cardiovascular, gastrointestinal, and central nervous system drugs. Meanwhile, drugs in oncology (figure 2), infectious disease, respiratory, and immunology TAs do not show a correlation. (See Appendix II for the figures for the other TAs).

Why does competition drive formulary positioning in some TAs and not others?
The level of unmet need, the cost to treat a patient population, the differentiation among drugs classes, the nature of the therapeutic, and the level of personalization are some factors that could be driving differences in how formulary positioning is determined. Comparisons between metabolic and oncology offer some insights into why competition might drive formulary positioning in one TA as opposed to another (figure 3).

Level of unmet need
Regardless of competitive intensity, drugs that treat diseases with high unmet medical need—conditions where treatment or diagnosis is not adequately addressed by existing treatments, such as cancer—are likely to have preferable formulary positioning that gives patients better market access. Existing treatment options for diabetes can significantly delay the progression of the disease, whereas patients suffering from some cancer types are still facing high mortality rates. In oncology, despite increasing levels of competition across drug classes, most drugs fall in Tier 2 or 3 (figure 2). However, in metabolic we see a wider range of formulary positioning for drugs as the level of competition increases, including a greater proportion of drugs that are not covered (figure 1).
Pharmaceutical cost to treat population
The pharmaceutical cost to treat a patient population is another factor that could influence the use of tiering to control market access. At the population level, the cost to treat diabetes, for example, is much higher than the cost to treat many cancer subtypes. This is driven by the much higher incidence of diabetes as compared to cancer types. In 2014, total prescription drug spending on cancer was US$10.9 billion, while for diabetes it was US$47.3 billion.1 Payers are more likely to use formulary tiering to control and manage market access for drugs that treat diseases that impact a significant portion of the population and have a bigger impact on their budgets. However, payers may use other tools to control and manage market access for drugs that are more expensive at the individual patient level, such as those that treat cancer (see the discussion on the nature of the therapeutic below).
Differentiation of drug classes
The number of treatment options and the differentiation in clinical outcomes among them can also influence how much competition drives formulary positioning. For example, there are more drug classes available to treat metabolic diseases such as diabetes and hypercholesterolemia than for certain cancer types. Among those drug classes, there is greater variability in patient response in cancer than in metabolic. For example, some cancer therapies are only effective on certain tumor types, whereas response rates for some diabetes drugs may be more consistent. In this context, payers may want to offer providers greater flexibility when determining what treatment option to choose for cancer patients.
In addition, clinical differentiation among branded metabolic drug classes and those that have already gone generic may not be that significant. The availability of cost-effective generics gives payers further leverage to use tools such as formulary positioning to control and manage market access and associated costs.
Nature of the therapeutic
The nature of the drugs in a TA in terms of their structure (small molecule or biologic), the way they are delivered (via specialty pharmacy or retail), and typical pricing can also influence the types of tools that payers use to control and manage market access. In oncology, many drugs are complex biologics that require special handling and are relatively higher priced. In contrast, metabolic drugs tend to be small molecules (though not always), are available in retail pharmacies, and are comparatively lower-priced. The nature of the compound also influences the availability of generic competition—the pathways to develop biosimilars are more complex than the pathways to develop generic small molecule drugs.
Our data suggests that payers tend to rely on pharmacy benefit designs such as prior authorization or step therapy more often for complex, specialty drugs than for small molecule drugs available through retail pharmacies. This is likely driven by the fact that many specialty drugs are relatively expensive, and payers may seek to implement controls to ensure that the patient receiving the drug is likely to benefit.
Our analysis suggests that competing branded drugs may be placed on the same tier, but prior authorization and step therapy are used to control market access instead. For example, Deloitte reviewed formulary positions and prior authorization language from select health plans for 18 immunology drugs. These drugs were generally in the same tier (typically tier 5). However, we found that plans used different prior authorization criteria and language for each in order to influence use of and, thus, spending on those drugs. The design of the prior authorization program cannot be determined by reviewing the formulary alone; one should analyze the specific prior authorization language to determine which products are advantaged or disadvantaged. However, prior authorization language is not standardized and can be complex to analyze and interpret across plans.
Degree of treatment personalization
Personalized, targeted therapies can help payers ensure appropriate use and increase the likelihood of market access. Companion diagnostics can help distinguish between patients who may benefit and those who might not or help identify who might be at higher risk for side effects.2 In our analysis, a cancer drug with a companion diagnostic used to treat lung cancer has a formulary score of 25.85, lower than the average score, 41.57, of two hypercholesterolemia drugs. The higher formulary score of the cholesterol drugs could be associated with the higher price relative to existing treatments, and lack of a way to ensure appropriate use. Without a companion diagnostic, payers might struggle to ensure that patients who would benefit from the drug are the ones receiving it. As a result, these drugs have experienced low acceptance even after proving their clinical superiority to the current standard of care.
What drives preferential positioning within drug classes?
Drugs in a class competing directly with each other typically have the same competitive intensity score but may end up with varying formulary positioning for different reasons, including clinical differentiation, nonclinical differentiation, and use of more strategic pricing and contracting levers, such as value-based contracts.
Clinical differentiation
Drugs that have greater clinical efficiency and fewer side effects often obtain preferential formulary positioning. Ideally, the drug with best patient outcomes would be preferred over others in a competitive drug class. For example, consider two hepatitis C drugs. Post-marketing data demonstrated that the risk of progression to decompensated cirrhosis is greater for patients who are treated with one drug versus those treated with the other.3 In our analysis, the drug associated with lower risk has preferential formulary positioning (formulary positioning score of 27.52) vs. the drug associated with higher risk (formulary positioning score of 48.66).
Differentiation beyond the product profile
Plans may be more willing to pay for drugs that provide more benefit to the patient. For example, a drug that is taken once weekly that has the same outcome as one that is taken once daily might offer greater patient convenience. Patient adherence is likely to increase, as is patient satisfaction with the product. In addition, patient services that help patients manage their medication and their disease could help drive better adherence and patient outcomes. These factors can be strong differentiators, especially as health care becomes more responsive to consumer demands.
For example, amongst a class of three type 2 diabetes drugs in our analysis that have the same competitive intensity score, the drug with the lowest formulary positioning score (27.36) has been shown to have better patient retention than others in the class.
Pricing and contracting approaches
Companies offering lower net prices through rebates typically obtain preferential formulary positioning as a result. Sometimes, exclusive contracts (in commercial plans) are offered in exchange for preferential formulary positioning.
We are starting to see signs that the market is shifting away from volume-based agreements and toward more value-based contracting. For example, 16 drugs in our analysis currently have or have had a value-based contract in place.4 More than half were for drugs in the metabolic TA, but others included drugs in oncology, cardiovascular, immunology, and infectious diseases.
In metabolic, the examples that we are seeing are primarily for new drug classes that are leveraging value-based contracts as a way to demonstrate value and encourage greater use. For example, a class of new cholesterol drugs introduced value-based contracts to help reduce clinical uncertainty around who would benefit from the drug. In addition, manufacturers of three drugs in a relatively newer class of diabetes drugs employed a similar approach to help demonstrate the class’s value beyond already established treatment options.
Different types of plans use tools to control market access differently
The use of formulary positioning as a tool to control and manage market access may differ depending on the plan type. The trends described above are specific to commercial plans, but other plan types may use tools to control market access differently. For example, while our data shows that both commercial and Medicare plans use tiering, prior authorization, and step therapy to control market access, we find differences in how they use these tools.
Looking at the entire set of drugs, it appears that Medicare plans use higher tiers than commercial and have a greater percentage of drugs that are not covered for some populations. In most commercial plans, most drugs fall in tiers 2 or 3. In most Medicare plans, the spread is much wider, with some drugs placed as high as tier 5 (figure 4). Drugs that appear to have even higher formulary positioning scores are those where a proportion of the population does have access to the drug. Medicare plans operate in a different regulatory environment than commercial plans, so that may be contributing to some of these differences.
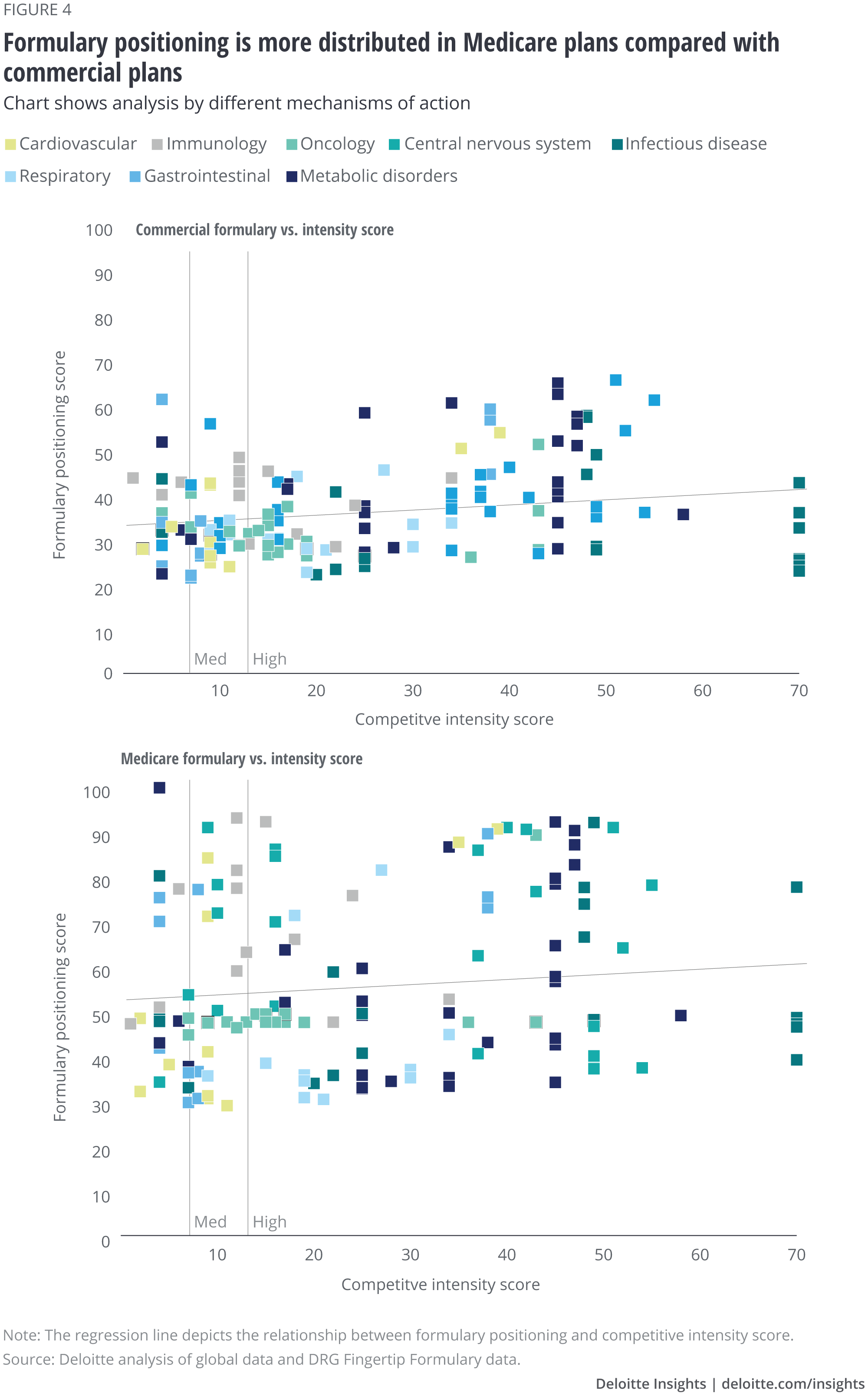
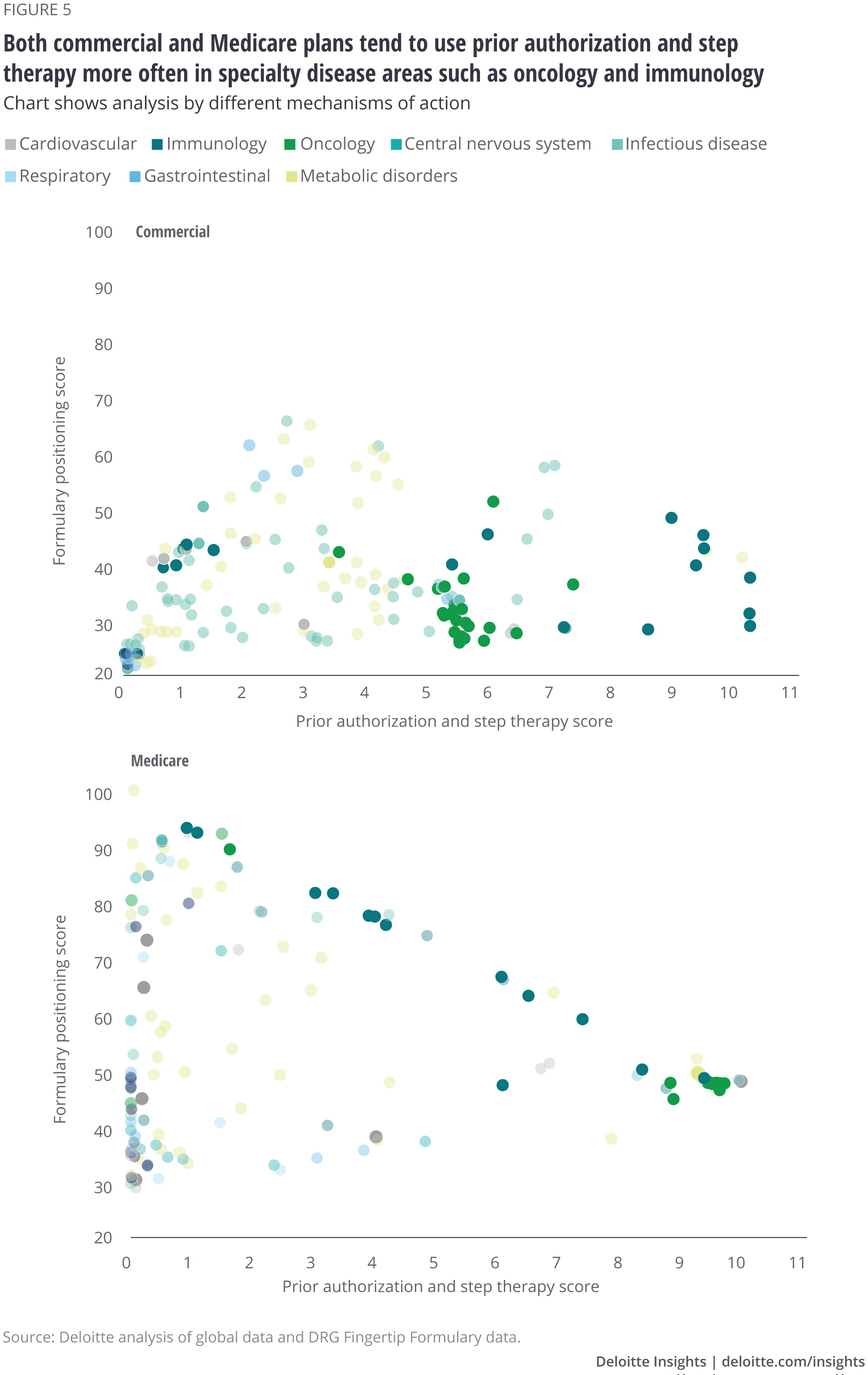
We also found differences in use of prior authorization or step therapy (figure 5). Commercial plans appear to use both on drugs across TAs, regardless of formulary positioning, while Medicare plans appear to use more prior authorization and step therapy for drugs on lower tiers (especially for specialty TAs) than those in higher tiers.
Both tend to influence utilization and market access by using prior authorization and step therapy more often in specialty TAs such as immunology and oncology. However, Medicare plans tend to rely on prior authorization and step therapy more heavily for drugs that are on lower tiers, especially in these TAs.
Understanding the differences between Medicare plans and commercial plans in how they use tiering, prior authorization, and step therapy to control access may help companies devise specific strategies for each stakeholder.
Uncertainty surrounds the future of the rebating system
Market dynamics around drug pricing are under scrutiny by policymakers, health plans, pharmacy benefit managers (PBMs), and other stakeholders. One debate on drug pricing centers around the rebate exchanges between PBMs, private companies that negotiate drug prices on behalf of payers, and drug manufacturers.
- Policy makers are scrutinizing the PBM industry and its practices around rebating. In January 2019, the administration proposed to change the safe harbor protections granted under the Anti-Kickback Statute to exclude protections for drug company rebates to PBMs and Medicare Part D and Medicaid managed care plans. It proposed to add a new safe harbor protection to allow drug manufacturers to reduce prices at the point of sale to consumers.5 The secretary of the US Department of Health and Human Services also has encouraged Congress to pass legislation that would bar similar arrangements in commercial plans.6 Even though as of July 2019 the proposal had been dropped, HHS leadership is still bullish on doing something about the rebating system.7
- PBMs are passing on rebates at the point of sale. Several large PBMs have announced they will begin passing 100 percent of the rebates they receive from drug manufacturers along to plan sponsors.
- Payers are establishing formularies that reduce the influence of rebates. One PBM has developed a formulary that attempts to shift away from products with high list prices and high rebates toward products with lower list prices.8
If the rebate system changes, manufacturers will need to consider how to adapt their market access and pricing strategies. For example, removing drug rebates and transitioning to a new model could accelerate the use of value-based contracts for prescription drugs if new safe harbors address some of today’s legal and regulatory hurdles. Future value-based contracts might need to take a different form since most have been designed in the context of today’s rebate programs.
At this point, it is unclear which, if any, of these policies will pass or gain traction from the administration and/or Congress. But drug pricing is likely to continue to be a focus for the administration as it focuses on advancing policies outlined in its Blueprint to lower drug prices and reduce out-of-pocket costs.9 Market access leaders should continually track these changes as they evolve and adjust strategies according to new market dynamics.
Recommendations
Optimizing market access requires an understanding of how payers might leverage tools differently among TAs or disease areas or across segments. Market access leaders should make decisions around what strategies to prioritize and implement in this context. Drug manufacturers can leverage several different strategies to influence or make the best of formulary positioning, including:
- Differentiating on clinical outcomes using real-world evidence. Evaluate additional outcomes to demonstrate superiority in class, improvements in standard of care, or efficacy in specific patient sub-populations.
- Incorporating “market access”-relevant endpoints in clinical-trial design. Leverage endpoints that are valuable to payers, which will help differentiate the value of the drug, such as overall clinical cost offsets.
- Learning how to execute in disadvantaged formulary positions. Achieve higher profitability through a less-advantaged position, and learn how to execute across the go-to-market model in that position (for example, better field execution).
- Differentiating based on patient preference. Differentiate based on patient convenience or by providing other patient services (for example, strategies that boost adherence).
- Patient support services. Offer solutions to help patients understand their disease, manage their medication, and navigate prior authorization requirements (especially in specialty areas).
- Targeted therapeutics. Aim to reduce payer budget impact by identifying target populations that receive the maximum benefit.
- Value-based contracting. Implement value-based contracts to help demonstrate the value of a new product or product class, or to mitigate the clinical uncertainty of a high-cost treatment.
Deciding what strategies to implement requires an assessment of the business case and ultimate impact on market share and profitability. For example, using rebates to improve the formulary positioning of a drug in an undifferentiated drug class could be expensive. Instead, companies could consider alternative strategies to increase market access for drugs that fall on higher tiers. For example, patient assistance programs have been used to support uptake in those situations; likewise, better field execution and targeted office support have also helped enable revenue generation in more difficult environments. These strategies could be less costly than increasing rebates to secure preferential formulary placement.
The relative prioritization of these strategies might change as the market continues to emphasize the value of pharmaceuticals. The rise of value assessors such as The Institute for Clinical and Economic Review (ICER), the increased use of value-based contracting, and public scrutiny of the rebating system are clear signals that the market is moving away from volume-based pricing and toward value-based pricing and contracting. In this context, market access leaders should increasingly consider what strategies will help tie price to value, including value-based contracting (see the sidebar, “Expanding the use of value-based contracts”).
Expanding the use of value-based contracts10
While the number of publicly announced value-based contracts between life sciences companies and payers has increased over the past few years, barriers continue to prevent widespread adoption.
Deloitte brought together thirty leaders from health plans, health systems, pharmaceutical manufacturers, and patient organizations to discuss some of the major barriers to greater adoption of these arrangements. All the participants agreed that one way to increase the adoption of value-based contracts would be for industry players to share the successes and failures of these arrangements more widely—that all stakeholders would benefit from greater transparency around what works and what doesn’t. Moreover, expanding the conversation to other stakeholders who play key roles—such as PBMs, large employers, regulatory agencies, and clinicians—could lead to greater success in the future.
Many organizations also lack the necessary infrastructure and resources to efficiently collect, link, and analyze the necessary patient data to support VBCs. This hurdle can make the implementation of the value-based contract too labor-intensive or lead to contract designs that are based on “what can we measure” instead of “what should we measure.” To achieve the necessary infrastructure to overcome the industrywide data challenge, stakeholders from across the health care industry could come together to develop a shared utility-like platform that can drive the necessary efficiencies and economies of scale to support VBCs. The advances in cloud-based technology, automation, analytics, and security make it possible to create a trusted, secure environment to design and implement VBCs. It can also bring transparency and objectivity into the programs and help break through barriers that exist today—especially around managing and analyzing complex real-world data sources.
In order to make meaningful progress on value-based contracting programs, providers, patient organizations, payers, and life sciences companies should collaborate to build the necessary capabilities, standards, and technology that will accelerate the shift to these new models. Doing so can help control costs, create access to care, and improve patient outcomes.
Market access strategy should underpin decision-making throughout the entire product lifecycle, including portfolio decision-making. Market access input is critical in the early stages of clinical design to help ensure that sufficient evidence is generated to support value claims and associated value messages.
Market access leaders could benefit from establishing a systematic, consistent, and cross-functional approach to building a strategic market access capability. Such an approach should guide decision-makers and their teams through a consistent set of choices and key business questions, including:
- Defining the product’s market access ambition and identification of trade-offs;
- Sizing the market potential, defining impacted patient populations, and identifying key stakeholders;
- Quantifying value attributes, pricing, contracting approach, and value narrative; and
- Implementing the access model and monitoring performance to ensure access effectiveness and results.
New automation technologies can accelerate and streamline certain tasks.
The days when market access organizations were perceived simply as trusted centralized contracting services are long gone. Deeper understanding of access issues and choices and the development of rigorous strategies can enable success in the market, throughout the entire product lifecycle.
Appendix I: Methodology
The analyses presented in this paper leverage two proprietary scoring systems developed by Deloitte researchers. We mapped the drugs with greater than US$100 million annually in US revenue for the top 19 pharmaceutical companies (by US prescription sales for 2016) to understand the competitive landscape and formulary pressure faced by the drugs. The revenue data was sourced from Global Data. We performed the analysis on 286 drugs.

Competitive intensity
To measure the competitive intensity a drug faces, we established a weighted scoring methodology that factored in the key variables shown in figure 6.
We then placed each of the drugs in a distribution to gauge where they landed on the competitiveness spectrum. The spectrum was divided into low, medium, and high based on a threshold number derived by factoring in the key variables mentioned in figure 6, with “X” being the intensity score of the drug:
- Low: If ≤7 points
- Medium: If 7 < X ≤14 points
- High: If >14 points
Approximately 46 percent of the drugs fall in the “high intensity” category, which is any drug scoring more than 14 points on our 70-point scale.
Formulary positioning
Using data from DRG’s Fingertip Formulary, we applied a scoring methodology to rank drugs according to their position on commercial and Medicare formularies. Drugs covered predominantly under the medical benefit (58 drugs) were excluded from our list. For formulary analysis, we considered 158 drugs from 8 therapeutic areas. Therapeutic areas considered are: metabolic, oncology, cardiovascular, central nervous system, gastrointestinal, immunology, infectious disease, and respiratory. The DRG database was used to categorize the drugs into classes based on their mechanism of action (MOA).
We calculated score subtotals for two factors and then combined them for a final “formulary positioning” score:
- Tiers: This score takes into consideration what tier(s) the drug is placed on by each type of payer.
- Not covered: We also considered the percentage of the population for which the drug is not covered.
Prior authorization and step therapy
Step therapy and prior authorization were considered separately. We calculated a weighted score that takes into account the percentage of the population for which the drug is subject to either or both of these types of pharmacy benefit designs.
Appendix II: Charts for additional therapeutic areas

© 2021. See Terms of Use for more information.
Read more about biopharma
-
Life Sciences Collection
-
The future of real-world evidence Article6 years ago
-
Survey finds biopharma companies lag in digital transformation Article6 years ago
-
How master protocols are changing drug development Article6 years ago