A new view on China’s digital health care Launching innovative biopharma in China
8 minute read
16 March 2019
Getting acquainted with China’s digitally savvy consumers means your innovative product launch can target them directly. Deloitte matches digital health care trends with opportunities in this second of a four-article series.
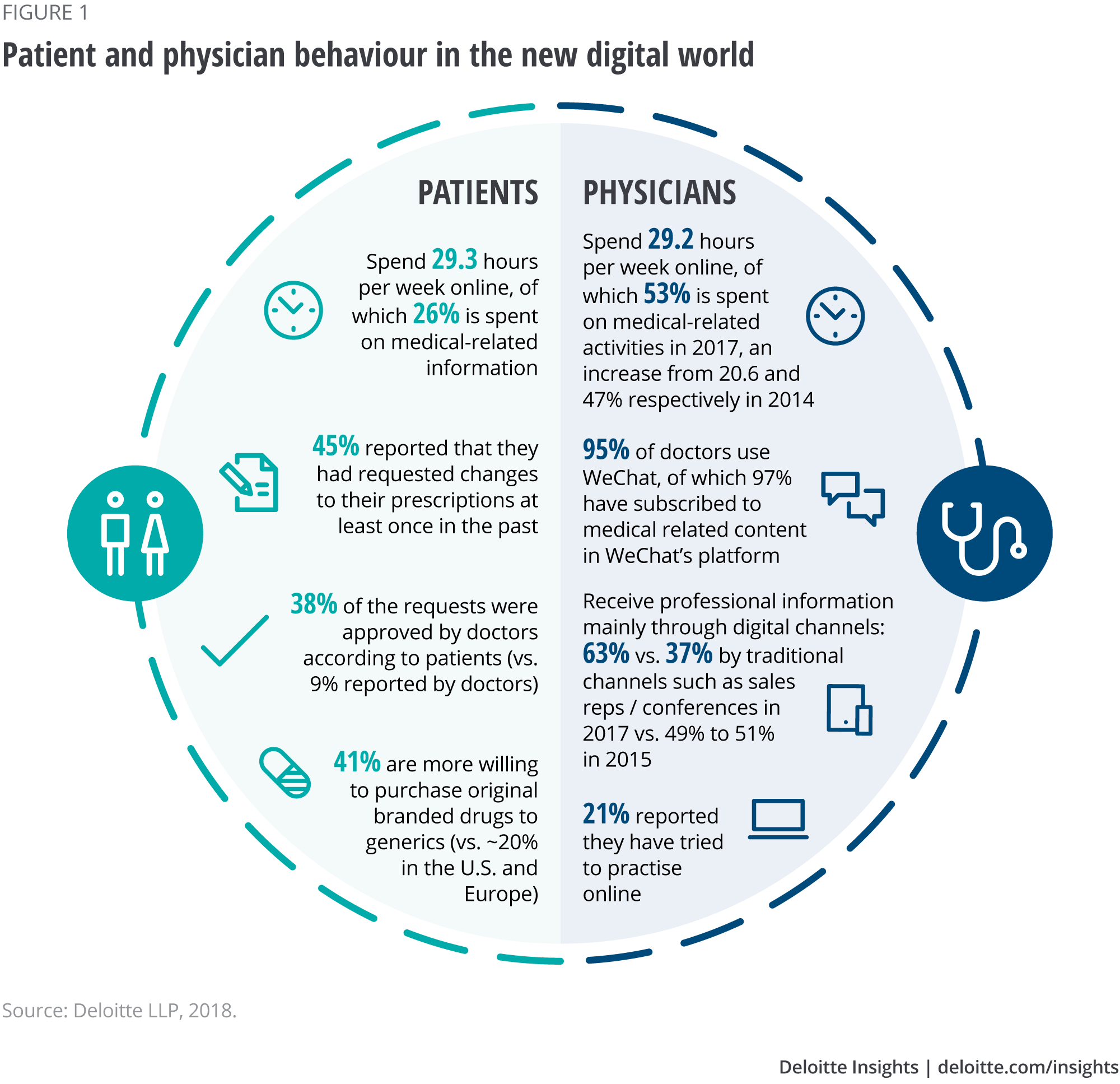
Traditionally, China is seen as driven by relationships and supporting a ‘face-to-face’ culture in business. But the country is now leading the way in the digital world, widely adopting new technology that ranges from mobile devices to innovative digital applications and tools.1 This has transformed many marketplaces, certainly including health care. The changes extend to customers: Patients and physicians are behaving differently, spending a significant portion of their average 29 online hours per week conducting medical-related activity (see Figure 1).
Managers of international biopharma companies and global manufacturers of biological and chemical medicines must get to know physician and patient behaviour in this new era. For teams looking to launch innovative medicines in China, it is not enough to invest in digital capability. Effecting a successful launch means targeting doctors and patients with the right content, through the right platforms using the right methods, while absorbing and exploiting a deep understanding of the fast-changing digital ecosystem.
Learn More
Explore the Launching innovative biopharma in China series
View the Life Sciences collection
Subscribe to receive more information about innovative biopharma in China
The connected patient and physician
Patients in China spend about 29.3 hours online every week, with 26 per cent of that time devoted to searches for medical information, according to the Social Media Trends Report 2017 by DXY Kantar (see Figure 1). Like their western counter- parts, Chinese patients now increasingly consider the internet an important source of health-related information. They rely on search engines and apps for information regarding their diseases, treatments and medications.
Patients are also taking a more active part in their own treatment decisions, rather than acting as passive recipients of information. Forty-five per cent reported that they have requested changes to their prescriptions at least once in the past, and 38 per cent say their requests were approved by doctors. (Doctors themselves estimate this figure as being only nine per cent.)
Similar to patients’ behaviour, doctors spend 29.2 hours online weekly, dedicating a little more than half of that (53 per cent) to medical-related activity. Among the digital channels, the Chinese app WeChat2 plays a major role: 95 per cent of doctors use it, and 97 per cent of those users have subscribed to medical-related content through it.
With easy access through smartphones and other devices, digital channels have grown to become the main way doctors receive professional information. In 2017, digital platforms were the source of 63 per cent of doctors’ information, compared to the 37 per cent they received from sales representatives and at conferences (see Figure 1). The previous year, the balance was 49 per cent and 51 per cent, respectively. Some doctors are also practising online: 21 per cent report doing so, for various reasons, such as building their reputation, generating additional income or finding potential patients.
Digital trends and China’s evolving health system
Among the many digital trends shaping global markets, Deloitte has identified four that will likely bring significant change to China’s health system in the short term (see Figure 2), as described below. They should also affect how biopharma companies plan a product launch (see the section Knowledge is power: Turning trends into advantage).
TREND 1: INFORMED AND DEMANDING PATIENTS MANAGING THEIR HEALTH
As shown by the data above, Chinese patients (and other health care consumers) are more informed about health care decisions, and are therefore engaging more in making them. Numerous consumer-facing digital platforms have emerged in the past decade, including digital health websites and interactive applications and platforms, such as virtual patient communities. These new tools not only change how patients gather and use information, but also offer new opportunities for the industry to influence patient behaviour and to continuously support them outside the traditional care setting.

For example, in November 2017, a big pharmaceutical company joined Ali Health3– a health care platform of the Alibaba Group – to introduce the Comfort at the Fingertips app. Users can take advantage of patient management programmes for chronic diseases. They can also find information about medication safety by scanning the barcode on a medicine package.
TREND 2: PHYSICIANS WITH NEW CHANNELS OF COMMUNICATION
The trend of Chinese physicians receiving more professional information digitally is set to continue. For one reason, the pharmaceutical industry is using digital tools to improve the efficiency of sales and marketing (particularly from a cost perspective). For another, regulators have tightened their grip on sales representatives, making digital channels a more viable way to communicate.
Biopharma and technology companies are learning how to engage physicians, either through in-house platforms, such as a big diabetes platform, which was built by a leader in innovating the therapy of diabetes and obesity; or through partnerships with technology companies that have already established digital health platforms, such as Chunyu Doctor. Virtual platforms are useful to offer high-quality medical education and training. Moreover, virtual conferences and live broadcasting have been widely adopted to replace traditional campaigns and events.
TREND 3: EVOLVING DELIVERY SYSTEMS
Traditionally, Chinese health care resources have been disproportionally concentrated in the large hospitals of major cities and metropolitan areas,4 and they are not keeping up with demand. Digital technology could help to ease the burden. Patients and health care providers in China are exploring alternative, more efficient care delivery models, such as virtual medical consultation. As in many Western markets, such digital health tools have been used to connect patients and doctors at a much lower cost, and have liberated health care system capacity. The biopharma industry has started to tap into this space, too. A big pharmaceutical company and Ali Health have begun a partnership to offer vaccination con- sultation online, aiming to optimise patient support and reinforcing the launch of innovative medicines, in China.5
From a logistical standpoint, digital apps could offer biopharma companies a new go-to-market model for alternative types of delivery. Such apps include direct-to-patient and so-called O2O (on- line-to-offline) pharmacies, through which online information and services enhance the customer experience. However, changing regulations have imposed a degree of uncertainty on this area, to support or sometimes to discourage online pharmacies. Traditional distributors are now actively exploring e-commerce opportunities.6 For example, the new government drug policy may allow bio-pharma companies to engage third-party logistics firms to deliver drugs; logistics enterprises may hence use the opportunity to expand the pharmaceutical logistics system, and connect with the pharmaceutical e-commerce supply chain to expand the third-party logistics market.
TREND 4: BIG DATA AND ADVANCED ANALYTICS
China is boosting its investment in big data and advanced analytics. In 2017 the disclosed funding in artificial intelligence (AI) start-ups rose to $7.06 billion, versus $5.62 billion for comparable US companies, according to CB Insights. 7In the health care space, with the same momentum, the industry has witnessed a spike in the number of investments and new entrants.
Major players like technology firms have begun to collaborate with hospitals to explore the potential of big data in health care.8 Ali Health has begun to work with a big multinational pharmaceutical and biopharmaceutical company, aiming to deliver new, smart health services driven by digital health data and AI.9 Within this fast-moving space, it is reason- able to predict that a new care model will emerge, supported by AI, analytics and new payment models, enabled by big data.
Knowledge is power: Turning trends into advantage
Clearly, biopharma companies should continue to invest in digital capability and exploit the trends above to support launch excellence. But can your innovative products achieve their full potential in the digital field? Below we recommend actions to mitigate four of the most common obstacles.
1. FORM A LONGER-TERM VISION/ VALUE PROPOSITION
Biopharma companies often lack a clear vision of digital technology’s role in a launch programme; sometimes they lack any overarching digital strategy. From an internal aspect, there are often different views within a launch team. Some members may believe in a fundamental shift toward a medical information-driven commercial model that uses multichannel marketing. Others may tend to rely on the traditional sales representative-driven commercial model, seeing digital tools as marketing support. From an external aspect, when it comes to partner- ships, brand teams often select digital partners based on current technology or channel needs, without a holistic, long-term vision for both partners.
To use digital technology effectively, clarify your long-term vision and the role of digital technology early on, within a brand or beyond, such as across the relevant therapeutic area or at an organisational level.
2. TARGET THE CUSTOMER WITH THE RIGHT CONTENT
In this era of explosive information, it is especially important to attract the time and attention of customers (physicians and patients): an endeavour that has become increasingly competitive. For an effective launch of innovative medical products, engage customers effectively with all the available channels. Your marketing team needs to be able to answer these key questions:
- What are our customers’ channel preferences?
- Have we assessed the channels that should be used, and which ones should we now prioritise?
- Which external partners are we ready to work with?
3. EMBRACE CROSS-FUNCTIONAL COLLABORATION
Frequently there is a lack of cross-functional collaboration, especially in digital health. Often, there is no follow-up or feedback collection after a digital marketing team sends out information to customers. Or, digital channels generate substantial new data – such as about key accounts or the extent physicians make online versus traditional prescriptions – but there is no analysis to make use of it.
To exploit the full potential of digital tools, liaise with the digital team in a launch’s planning stages, and aim to form an internal system of collaboration. Integrating digital programmes will help achieve the best outcomes and transform patient pathways. For example, you can build better data and service relationships, to improve local health economy patient outcomes. You can also efficiently collect data for outcomes-based contracting and a deeper understanding of how therapies are used.
4. SUPPORT SYSTEMATIC DIGITAL PROCESSES
Some biopharma and medical device companies have not established the optimal internal structure to support systematic digital processes, such as a robust digital infrastructure, a centralised data registry and processing platform, or a dedicated digital project team. Companies often respond to the fast-moving market by setting up ad hoc digital teams, with limited dedicated resources and unclear processes and governance. Digital processes are sometimes de-prioritised or merely seen as a showcase for a ‘trendy way’ of marketing; in these cases neither the right talent nor structure is in place to support a launch or other commercial activities. Again, companies should ask themselves vital questions:
- Do we have a defined model for digital processes and governance?
- Are we prepared to hire new talent?
- What internal structure will we set up to drive the success of our launch?
- How should we design frameworks and guidance to ensure that various teams are aligned?
Conclusion
A deep understanding of the fast-changing digital ecosystem is becoming increasingly important for biopharma companies planning launches in China. It is also critical to understand the tendencies and needs of physicians and patients in this environment, to best engage them with the right content, channels and methods.10 Recognising areas where your strategy needs work should redirect your launch team toward a structured, systematic and pragmatic approach – one that facilitates the development of a multichannel marketing programme. Taking full advantage of all available digital opportunities can give your product the edge it needs to cut through the noise.
Explore further insights into launching innovative biopharmaceuticals in China with three other articles in this series, on: digital technologies, regulations and the changing health care environment.
© 2021. See Terms of Use for more information.
Read other articles in the series
-
Gain the edge in a fast-moving market Article6 years ago
-
A new view on biopharma market access in China Article6 years ago









