Analysis

2021 global life sciences outlook
Possibility is now reality, sustaining forward momentum
Navigating the pandemic has been an all-encompassing, once-in-a-lifetime challenge. Globally, life sciences companies responded with leadership and are emerging stronger. How will life sciences companies continue to respond and what areas can they build resiliency going forward?
Explore Content
- Download the report
- Infographic
- Redesigning work, workplace, and workforce
- Accelerated digitization
- New customer-centric commercial model
- New types of collaborations and clinical trials
- Shortening development and review timelines
- Cross-border reliance
- Advancing humanity
- Explore previous outlooks
- Follow us on social media
- Key contact
Overview
The life sciences sector has played a pivotal role amid the COVID-19 pandemic. To cope with the global crisis, traditional competitors partnered to accelerate research and develop the fastest novel vaccine in the history. Governments, health systems, payers, retail pharmacies, and nonprofits are now working collaboratively with the sector to provide widespread distribution and administration.
With the introduction of this ‘new-normal’, digitization is broadening the horizon of new possibilities in the life sciences sector. Redefined workplace environments; the shift in health care delivery; and innovative collaborations to create efficiencies are a few examples that are leading to this unprecedented change supported by technological advancements. While pharmaceutical innovation is saving the world, now is the opportunity for biopharma and medtech companies to sustain this forward momentum.
In our 2021 Global Life Sciences Outlook, we explore the various ways COVID-19 accelerated change within the sector, the changes that are likely to stay, and what can be reimagined and made better. It also explores different scenarios for stakeholders to analyze how these changes can better transform the sector.
Global life sciences sector issues in 2021
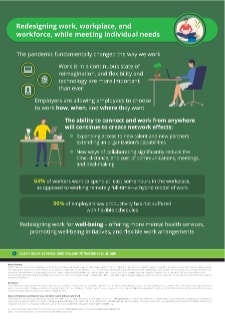
Redesigning work, workplace, and workforce, while meeting individual needs
COVID-19 has fundamentally changed our personal and professional lives. With life sciences companies working from remote locations, workplaces are being reimagined from virtual workspaces to new types of off-site collaboration. Life sciences companies are focusing more on individual needs of employees and promoting well-being. This growth in greater connectivity and the blurred geographical boundaries is also widening the pool of talent and access to skills.
Key takeaways:
- Organizations should take a more in-depth look at human resources and its evolving new role—encompassing the entirety of work, workplace, and workforce.
- The ability to connect and work from anywhere will continue to create network effects—expanding access to new talent and new partners, while extending an organization’s capabilities.
- It is essential to foster a culture of belonging and innovation for remote workers and redesign work to incorporate true well-being—this will be critical for workforce to thrive and acquire new talent.
- While new agile spaces designed specifically for teams to work and socialize off-site will grow to accommodate a post-COVID world, life science companies could think about repurposing space to drive client innovation.
Accelerated digitization: New points of care, new roles for pharma and medtech
Corporate funding for digital health reached a record US$21.6 billion globally in 2020—an increase of 103% over 2019. One thing is clear—with the help of digital health tools, virtual care can fundamentally change health care access and deliver an improved care experience. Digitization in the life sciences sector has also led to an increase in new point of care systems, digital pharmacy setups, and easy and efficient access to health care.
Key takeaways:
- Stakeholders are likely to continue funding new digital health innovation in the next year. Health systems are expected to continue making investments and a hybrid model of virtual and in-person visits will likely be the norm post-pandemic.
- Eased regulations during the pandemic increased adoption of telemedicine in many countries, but reimbursement and regulatory policies post-COVID will be key to permanent uptake and growth.
- Digitization has led to new points of care such as easy access to clinical care from home, retail and digital pharmacies, pharmacy vaccinations, drone deliveries, and more.
- The home is a hub of connectivity and emerging as a clinical point of care. Home testing and point-of-care testing are undergoing a revolution, and medtech companies can expect continued competition from consumer tech.
New customer-centric commercial model: Meeting physicians where they are, on their terms, and through more meaningful interactions
As the pharma commercial models are undergoing virtual shift, it is necessary for companies to focus on the needs of the health care providers (HCPs). Remote selling soared in 2020 as doctors and hospitals resorted to virtual meetings with sales reps. Companies conducted more than 316,900 remote meetings with doctors globally in April 2020. However, in the future, even this change is subject to the post-COVID requirements of each country. Companies will also focus on driving value to through digital channels to educate health care providers in the post-pandemic world.
Key takeaways:
- Future interactions between companies and HCPs: While a hybrid commercial model with a mix of in-person and virtual meetings is evolving, cultural differences will play a key role.
- As companies lean on to virtual models to engage HCPs now, digital and virtual interactions require new skills to deliver more personalized and empathetic experiences.
- Medical affairs will see more investment and strategic partners to complement its expanding roles and responsibilities.
- The mega medical congresses of the past are expected to make way for more virtual, focused, and hybrid physical/digital events, and will see growth in technologies such as artificial intelligence, chatbots, virtual rooms, and learning platforms.
New types of collaborations and clinical trials reshaping research & development
It’s believed that COVID-19 accelerated digital transformation of the pharma sector by several years. During the pandemic, life sciences saw agility, increased speed to market, and greater efficiencies. While the sector average for a new drug development and review is 8.2 years, the two novel COVID-19 vaccines were developed, tested, and authorized in less than a year. As a result, companies are reassessing and challenging their previous processes to enhance efficiencies. They are also looking at innovative partnerships to excel. Additionally, companies are moving towards virtual trials and remote monitoring to involve more patients into their studies via telemedicine and mobile health care.
Key takeaways:
- Biopharma companies are adopting various strategies for innovating clinical trials to shorten timelines—including new trial designs, new technologies such as AI, and new strategic partnerships.
- New virtual trial designs enable greater patient involvement and improve clinical trial diversity to draw more valuable insights.
- Planning and resources are key to the success of these new models, especially to address the needs of the vulnerable or marginalized populations.
- Biopharma companies should work collaboratively and transparently with data startups to unlock the data necessary to inform transformative approaches in drug development.
Shortening development and review timelines, thinking more like a regulator
Due to COVID-19, regulatory authorities accelerated their drug approval processes. Rolling reviews for vaccines was one of their expedited regulatory tools for emergencies that also includes rapid scientific advice, accelerated marketing authorizations, and compassionate use programs. Developers were allowed to use platforms approved in other areas, such as mRNA, for new development, provided they had the data to support it.
Vaccines forced a global population health perspective, and the approach has been very collaborative between regulatory agencies and industry. Cross-agency scientific resources enabled shorter review timelines for COVID-19 drugs and treatments.
Key takeaways:
- Novel strategies and technologies, including real world evidence, platform trials, remote clinical trial monitoring, and advanced analytics can help organizations succeed in the new regulatory environment, compress timelines, and accelerate insights.
- Continued collaboration between governments, industry, and big techs will likely fund innovative technologies for widespread diseases based on the model that evolved during the pandemic.
- Regulators are taking a risk-based approach in everything they do and thinking like a regulator is key. The best way to do this is to study inspections and processes from the regulator’s perspective while analyzing risks and benefits carefully.
Cross-border reliance intensifies the need for supply chain visibility and reshoring options
COVID-19 shined a light on cross-border reliance—in trade, manufacturing, and distribution. To decrease foreign dependencies, there is a growing trend for bringing production back onshore, or reshoring. To meet demand and accelerate time to market, vaccine manufacturers produced vaccines at-risk. Further, special handling requirements for vaccines using mRNA technologies presented new challenges and required new solutions. Although companies believed they had resilient supply chains before the pandemic, what new lessons can be learned about resiliency?
Key takeaways:
- Organizations should evaluate their supply chain plan holistically and include their strategic, operational, and financial leaders to optimize resiliency. In 2021, a significant amount of investment is expected to continue in reshoring, especially for capacity shortfalls (e.g., injectables, vaccines).
- Changes in manufacturing and supply chain are driving the need for new partnerships. Increased collaboration between competitors is expected to ensure consistent and expedited delivery of drugs and vaccines to the public.
- Contract manufacturing agreements are growing for next generation therapies. A lot of pharma companies will need to leverage their partners instead of manufacturing the products themselves.
Advancing humanity: Environment, Social, and Governance (ESG) imperatives
In 2021, being a good corporate citizen by leading with purpose and advancing humanity will be an essential part of overall organizational success. Many industries experienced rapid changes in reputation during the pandemic, but none perhaps as drastic as the life sciences sector. With the sector on its front foot, how can companies build more trust and drive more growth for good?
Key takeaways:
- Organizations can lead by example, operate with integrity, build a secure foundation, deliver on patient-centricity, and innovate without borders, and embed trust into their DNA.
- Measuring ESG progress is critical to building trust and advancing the sector’s role in society. Business ethics, product governance, and access to basic services are key issues that could impact valuations of Big Pharma and biotech companies.
- An increasing number of pharmaceutical companies are shifting to electric vehicles (EV) as a force for positive change. Reducing pollution through pharmaceutical production is just one of many areas the sector is addressing.
- Organizations should focus on achieving equity in health, race, and gender. Companies are looking to invest in solutions which reduce inequity and drive meaningful change in the society. They are also expanding their resources network to break the gender and racial barriers and inequalities, while holding themselves accountable for their actions.
- Socially responsible companies are creating new key performance indicators (KPIs). Success today and in the future depends on collaborating for shared value in a way that is transparent and sustainable.
Read our 2021 Health Care Outlook to know about the issues driving change in the health care sector and present questions and actions health leaders should consider in the coming year.
Explore our previous outlooks
Review or download previous life sciences sector outlooks.
2020 global life sciences sector outlook
2019 global life sciences sector outlook
Explore Content
- Download the report
- Infographic
- Redesigning work, workplace, and workforce
- Accelerated digitization
- New customer-centric commercial model
- New types of collaborations and clinical trials
- Shortening development and review timelines
- Cross-border reliance
- Advancing humanity
- Explore previous outlooks
- Follow us on social media
- Key contact









Join the conversation