Legal representation set out in the EU Clinical Trial Regulation has been saved

Blog
Legal representation set out in the EU Clinical Trial Regulation
The European Commission hopes to create a favourable environment to conduct clinical trials in the EU with the implementation of the Clinical Trial Regulation (CTR), which is expected to go live at the beginning of 2022. In this blog we provide an overview of the key considerations with regards to legal representation under CTR.
By Paulien Nuyts, Bob Stoffels, Sara Teiken and Sebastian Payne
Go directly to
Background
With the implementation of the Clinical Trial Regulation (CTR) (No 536/2014), the European Commission hopes to realise its ambition to create a favourable environment to conduct clinical trials within the EU. The CTR is the new regulation that governs all interventional clinical trials conducted in the EU with medicinal products for human use. CTR aims to harmonise submission and assessment processes, improve cooperation and transparency in and between Member States and enhance overall safety standards. In this series of blogs, we will examine the key changes and challenges faced and the potential competitive benefits for organisations that CTR introduces. In this second blog, we provide an overview of the key considerations with regards to legal representation under CTR. Interested in a general introduction into CTR? Please read our first blog “Introduction to the Clinical Trial Regulation.”
Legal representative requirements under CTR
According to Article 74 of the CTR, if a sponsor of a Clinical Trial is not based in the EU, then they are required to appoint a representative within the EU to act as a Legal Representative. If the sponsor is based in the EU, then a legal representative is not required under CTR, because the organisation based in the EU will act as legal representative (see Figure 1). The legal representative ensures compliance with the Sponsor’s obligations under EU CTR and notifies the sponsor immediately in the case of becoming aware of incompliance with EU CTR.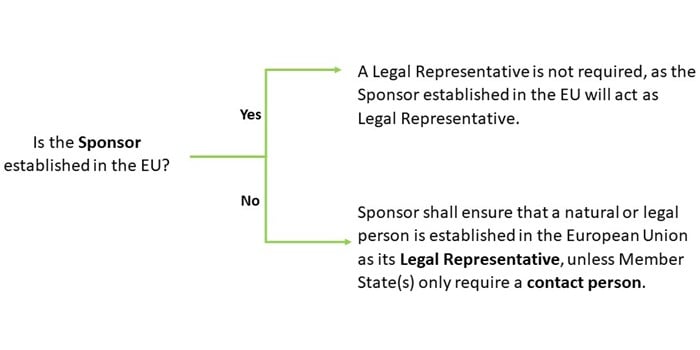
The Legal Representative is accountable for the sponsor’s compliance with obligations under EU CTR and is the addressee for all communications with the sponsor provided for in the Regulation. However, Member States can choose not to require a legal representative for clinical trials that are conducted on their territory or on their territory and that of a third country as long as they establish at least a contact person in respect of that clinical trial for all communications with the sponsor. If the clinical trial is conducted in more than one Member State for example in multi-territory trials, then all of the Member States concerned can choose to only apply a contact person established in the Union.
However, from our research it is becoming evident that the majority, if not all, Member States will require a Legal Representative instead of a simply a Contact Person for organisations not established in the EU.
Key considerations for organisations
With many organisations currently assigning a sponsor outside the European Union, the CTR presents a challenge. Below we present several options for organisations to comply with the requirements for a legal representative.
Possible approaches to meet the requirements for legal representative:
1. Continue with the current set-up
Depending on the internal organisational structure, this option is only applicable for organisations that either have their clinical trial activities in-house or their sponsor and legal representative are firmly based within the European Union.
2. Outsource the legal representative
Another option would be to outsource the legal representative, either by contracting the sponsor as well as another external party. This option may however be undesirable, as outsourcing the position of legal representation effectively means outsourcing the organisations compliance with the CTR. This option therefore creates full reliability on this party for the organisations representation, responsibility and liability within the EU.
3. Establishing an EU-based location or using an EU-based affiliate
An organisation may also opt for establishing an EU-based location or using and EU-based affiliate. This can be seen as a preferred option in case the sponsor is based outside the EU. The legal representative can then be based in the EU-based location or affiliate.
4. Establish a single central resourced organisation
Another option is for organisations to establish a single central resourced organisation under which all clinical trials within the EU are managed and executed. The single central resourced organisation would include the role of both the sponsor and legal representative. This would be beneficial, as a legal representative would not be required as the sponsor is based in the EU.
Relevance for organisations
As the requirement of legal representative is an essential part of the CTR, it is important for organisations to be aware of their current organisational setup and how they use external organisations (e.g. Contract Research Organisations (CRO)) in order to decide what approach is best suited. The choice of approach and organisational setup can also be influenced by access and user management within the Clinical Trial Information System (CTIS) as introduced by CTR. We will discuss the considerations around user management in our next blog.
How can Deloitte support you?
We have extensive experience in supporting regulators and top pharmaceutical companies to prepare for new Life Science Regulations, and since 2014 we have been helping organisations prepare for the implementation of the Clinical Trial Regulation. We are skilled with differentiating and setting up regulatory frameworks in challenging and diverse jurisdictions. We pride ourselves in supporting organisations in defining and executing optimal strategies for CTR implementation and ensuring they are and remain responsible businesses. Want to know more? Find our contact details below.


