Data Governance in Life Sciences has been saved

Solution
Data Governance in Life Sciences
Enabling governance and management of data in line with data standards
Evolving to a data-driven organisation requires data that can be trusted, across the entire organisation. Iperion is eager to support your evolution and ensuring you get more value from your data.
Your Challenges
Since the introduction of XEVMPD, and now with the implementation of IDMP, DADI, CTR and MDR, the demand for structured product data for regulatory submissions within Life Sciences continues to increase. Managing all this data, spanning multiple functions, as well as keeping up to speed with ever-evolving data standards requires strong data governance.
In order to evolve to a data-drive organisation, you will need accurate, reliable and consistent data across the organisation. Data governance ensures that your medicinal product data is of high quality, in accordance with data standards and also fit for purpose.
Next to compliance, data governance leads to major internal business benefits such as better data analytics and increased business efficiency (by not having to reformat or rework data), therefore shorter time to have fit for purpose data available.
Data governance is control and oversight over your organisation’s data management and datasets, including roles, responsibilities, and processes for ensuring accountability and ownership. A solid data governance framework enables an organisation to manage its data as a strategic asset.
Why Iperion – a Deloitte business
We can support the set-up of a data governance framework and/or accelerate and improve your maturity to ensure you get an increased value from your data.
We do this, by:
- Identifying how to get more value out of data in the most efficient way
- Defining and agree on who owns data, performs data maintenance and consumes data
- Setting up a Data Governance strategy & organisational structure
- Implementing Data Quality framework and data quality rules to increase data quality
- Improving an already established Data Governance organisation
Our Solution
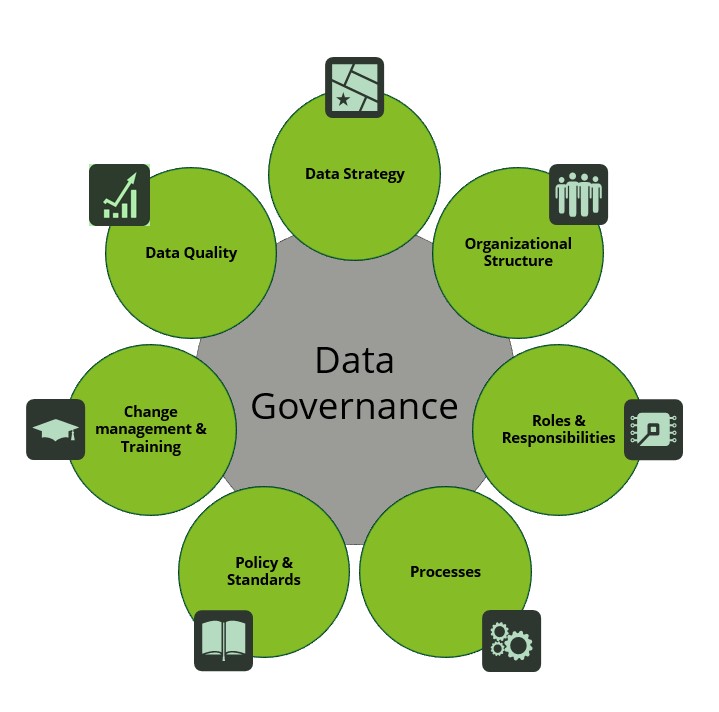
Our Data Governance framework consists of the following components:
Data Strategy
Define and implement a clear strategy with concrete goals defined, this includes a governance charter, an operating framework, data scope and an implementation roadmap.
Organisational Structure
An organisational chart, including an interaction model, is defined together with all required Data Governance functions and governing bodies.
Roles & Responsibilities
Interrelated role descriptions, linking to the organisational structure with clearly defined roles & responsibilities for the different Data Governance roles, to facilitate ownership and accountability.
Policy & Standards
Data Governance Policy that covers all key principles/aspects of data management used in operations of Data Governance. Sources of origin are identified, data standards & data definitions are established and relationships/interfaces are mapped.
Processes
Data management processes (describing how data needs to be managed) are defined covering all principles/aspects of the Data Governance policy, to be operational in all affected business functions. Data Governance Processes are defined to ensure that data is managed. Communication processes are established as part of the Data Governance framework and will facilitate communication of updates to policy, processes, standards and reporting.
Change Management & Training
Identify, engage and train a broad range of stakeholders to instill a new data culture in the organisation, including regular communication of Data Governance processes; Designed with specific users in mind and targeting awareness for the importance of Data Governance.
Data quality
Establish Data Quality framework and define Data Quality rules. Ensure Data Quality is actively monitored, improved and that Data Quality issues are effectively remediated.
Our Data Governance framework allows your company to establish correct data ownership, with associated Data Quality rules, ensuring data quality, enabling interoperability of data between systems and being able to respond to changing (Health Authority) data requirements.
Recommendations
Regulatory Life Sciences webinars Iperion
A total overview of all previous and upcoming webinars
Leveraging on effective Data Governance
5 typical challenges and 4 benefits of effective Data Governance