3D opportunity in medical technology has been saved
3D opportunity in medical technology Additive manufacturing comes to life
29 April 2014
Additive manufacturing capabilities align well with the needs of the medical device segment, enhancing product customization and enabling efficient, cost-effective production and delivery.
AM and medical devices: A natural fit
Want to learn more about 3D printing?
Visit the 3D Opportunity collection
Register for our upcoming course
The medical technology (medtech) industry has been a leader in the use of additive manufacturing (AM), also known as “3D printing.” In 2012, medical applications accounted for 16.4 percent of the total system-related revenue for the AM market.1 A key reason for this is that AM capabilities align well with the needs of medtech’s medical device segment. For example, the medical device segment serves a broad, geographically distributed population of service providers that in turn serves an even larger end market of health care consumers.2 Many medical devices, such as hearing aids, dental crowns, and surgical implants, are relatively small in size and therefore suitable for the production envelope sizes available through common AM systems.3Furthermore, these products are value-dense—that is, they combine relatively high value with relatively small physical volume—and the high level of customization available with AM makes this technology well suited for custom-fitting products to individual patients, an important factor in clinical efficacy.
AM capabilities align well with the needs of medtech’s medical device segment.
The medtech industry is also relatively well funded, which gives it the resources to invest in new technologies. The industry’s 2012 revenue was estimated at $121.6 billion, with an annual expected growth rate of 5.4 percent.4 The 15-year total shareholder return (1998–2012) for medtech companies was 7.8 percent, compared with the Standard & Poor’s 500 average of 5.2 percent.5 But to sustain this performance, the industry needs to continue to deliver innovative solutions to address patient needs.6
Given the strong alignment of AM capabilities with the medical device segment’s needs, and the medtech industry’s ability to support investment in new technologies, it is perhaps no surprise that AM has made substantial inroads with health care practitioners and service providers. Our goal here is to investigate ways that AM may influence the trajectory of the medical device segment.
Additive manufacturing is “a process of joining materials to make objects from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing methodologies.”7 The overall market size for the AM industry was estimated to be $2.2 billion in 2012, with a compound annual growth rate of 14.2 percent.8
Medical technology is a subset of health technology that covers products that diagnose, treat, and monitor human diseases.
Four tactical paths
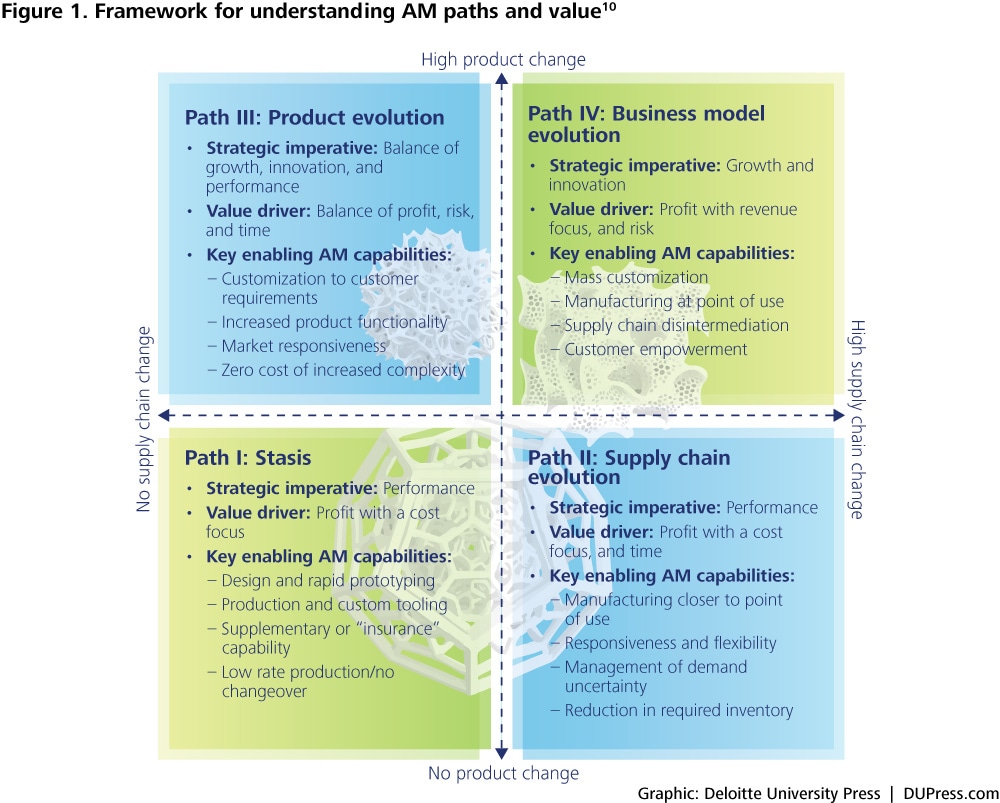
 Our view of the strategic impact of AM relies on understanding the ways in which the technology breaks trade-offs between capital and economies of scale and scope. Based on this understanding, we have developed an AM framework that identifies the tactical paths companies can follow as they seek business value using AM. This framework is summarized in figure 1.9
Our view of the strategic impact of AM relies on understanding the ways in which the technology breaks trade-offs between capital and economies of scale and scope. Based on this understanding, we have developed an AM framework that identifies the tactical paths companies can follow as they seek business value using AM. This framework is summarized in figure 1.9
AM is an important technology innovation whose roots go back nearly three decades. Its importance is derived from its ability to break existing performance trade-offs in two fundamental ways. First, AM reduces the capital required to achieve economies of scale. Second, it increases flexibility and reduces the capital required to achieve scope.
Capital versus scale: Considerations of minimum efficient scale shape the supply chain. AM has the potential to reduce the capital required to reach minimum efficient scale for production, thus lowering the barriers to entry to manufacturing for a given location.
Capital versus scope: Economies of scope influence how and what products can be made. The flexibility of AM facilitates an increase in the variety of products a unit of capital can produce, reducing the costs associated with production changeovers and customization and/or the overall amount of capital required.
Changing the capital versus scale relationship has the potential to impact how supply chains are configured, while changing the capital versus scope relationship has the potential to impact product designs. These impacts present companies with choices on how to deploy AM across their businesses.
The four tactical paths that companies can take are outlined in the framework below:
See endnote 10
Path I: Companies do not seek radical alterations in either supply chains or products, but may explore AM technologies to improve value delivery for current products within existing supply chains.
Path II: Companies take advantage of scale economics offered by AM as a potential enabler of supply chain transformation for the products they offer.
Path III: Companies take advantage of the scope economics offered by AM technologies to achieve new levels of performance or innovation in the products they offer.
Path IV: Companies alter both supply chains and products in the pursuit of new business models.
Using this framework as a lens, we reviewed academic literature and case studies and interviewed medtech and AM veterans to identify key current and future trends that are expected to shape the application of this technology within the medical device segment. As AM continues its rapid growth, applications are bound to change; however, this analysis represents our current view of the evolution of AM technology within the segment.
On path I, companies do not seek radical alterations in either supply chains or products, but they may explore AM technologies to improve value delivery for current products within existing supply chains.
Path I: Stasis
AM has historically been used within the medical device sector to improve performance in product modeling and prototyping, thus positioning most companies along path I of our framework. Companies on this path have used AM to improve product quality, reduce cost, and reduce time to market.11 The pursuit of these goals requires little alteration to a company’s existing supply chain or products, thus offering a significantly lower-risk entry point for firms looking to apply this technology across their operating model.
Companies on this path have used AM to improve product quality, reduce cost, and reduce time to market.
As an example, consider Kablooe Design, an invention, design, and engineering firm that helps companies evolve ideas from the concept stage through manufacturing. For Kablooe, creating products that help advance the medical industry is nothing new. Nearly 75 percent of the firm’s creations are medical devices.12 In one case, Kablooe was tasked with creating a device that would treat benign prostatic hyperplasia (BPH), commonly known as an enlarged prostate, in a less invasive way. More traditional BPH treatment is highly invasive and usually involves surgery (and thus a short hospital stay), bleeding, and scarring.
While developing the BPH device, Kablooe turned to the flexibility and short turnaround times of AM-enabled rapid prototyping to take the product from concept to end-product manufacturing. Kablooe estimated that it would take 10 iterations to perfect the device’s design.13 With this in mind, the company concluded that using traditional tooling and injection molding for prototyping would be too costly and time-consuming. By turning to rapid prototyping using AM, Kablooe was able to save more than $250,000 and 12 weeks of development time over the cost and time of using traditional manufacturing techniques. As an added benefit, the shorter development timeframe allowed Kablooe to collect feedback from practicing physicians and “tweak” designs in order to better meet their preferences.14
Orchid Design (Orchid) offers another example of a classic path I application of AM for rapid prototyping. A division of Orchid Orthopedic Solutions, Orchid is dedicated to working with medical professionals to prototype and test new orthopedic solutions. The development process, from initially sketching a proposed device through end-unit production, can take months of highly detailed design and process work, capped with the uncertainty of approval by the US Food and Drug Administration (FDA).15 Historically, Orchid had minimized its use of prototypes because of the high cost and time-consuming nature of orthopedic design, relying instead on computer-aided design (CAD) drawings. But the reliance on CAD meant that design flaws sometimes went undiscovered until later in the production process, when they were expensive and time-consuming to fix.
Pursuing a higher-performing development process, Orchid turned to AM to print high-resolution prototypes in-house. This allowed the company to increase the quality and manufacturability of its designs as well as reduce the development timeline, enabling Orchid to drive more revenue and increase repeat business.16 Rapid prototyping has also been helpful to Orchid in determining the functional requirements of small parts commonly used in the medical field. Through the use of AM, Orchid is now able to print parts at five to ten times their actual size to analyze how they will function, enabling the company to make adjustments to the design if necessary.17
Most medical device companies currently apply AM in the basic applications described above. This low-risk approach has allowed these firms to begin to adopt this technology without employing an overly aggressive strategy.
On path II, companies take advantage of scale economics offered by AM as a potential enabler of supply chain transformation for the products they offer.
Path II: Supply chain evolution
Path II activities focus on improving performance through the transformation of a firm’s supply chain. The majority of the potential benefits of pursuing this path come from the ability to reduce working capital requirements and reduce the minimum efficient scale of production.18
Historically, medical device companies have achieved less on path II than on other paths. Those that have pursued supply chain evolution through the use of AM have done so by focusing on two primary types of value: supply chain efficiency and inventory distribution.
AM drives supply chain efficiency
By streamlining a product’s supply chain, companies can reduce production costs, decrease the time it takes for a customer to receive the end product, and simplify multistep production processes. Firms concerned about their supply chain competitiveness may use AM to serve existing customers more efficiently and improve their service delivery capabilities.
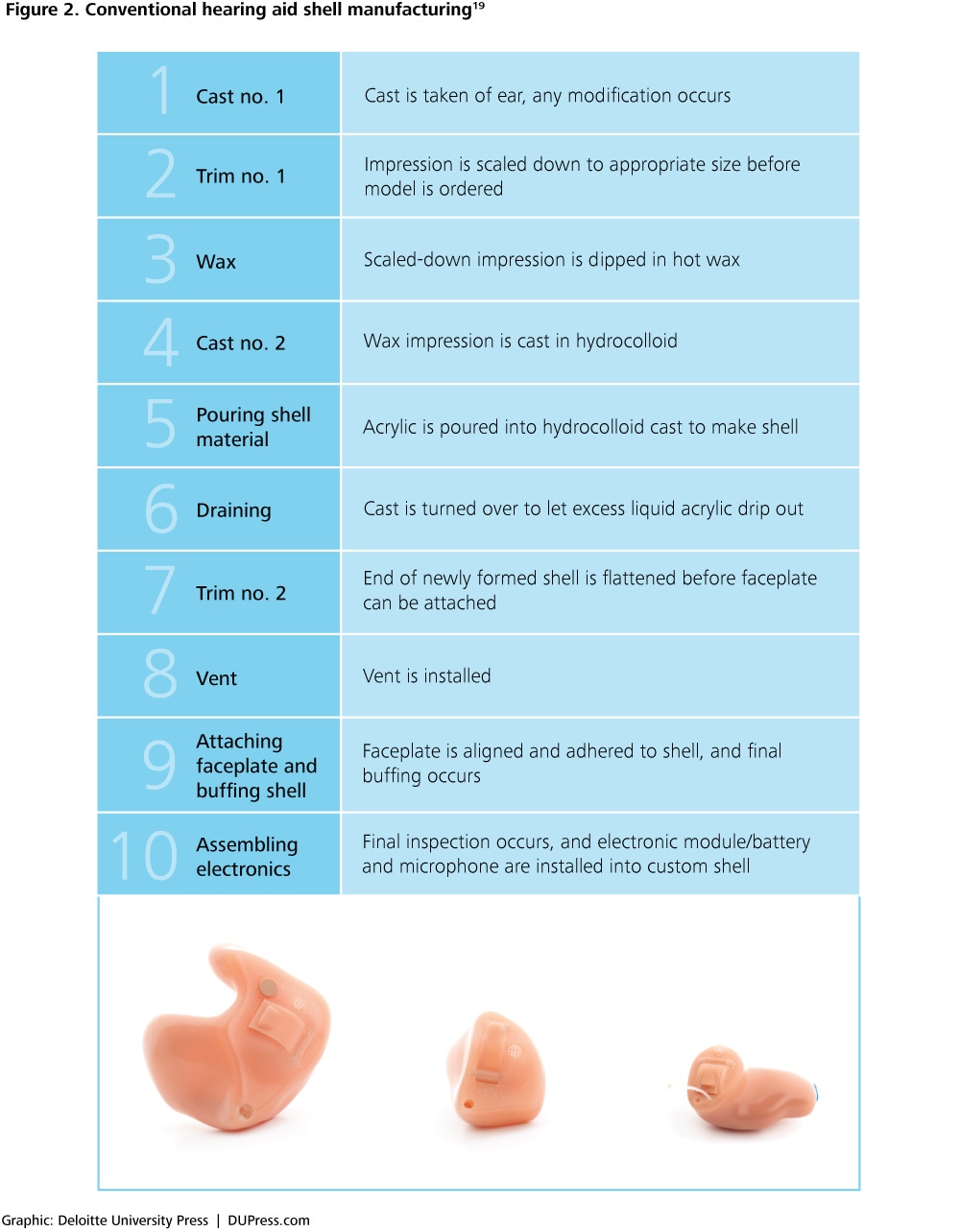
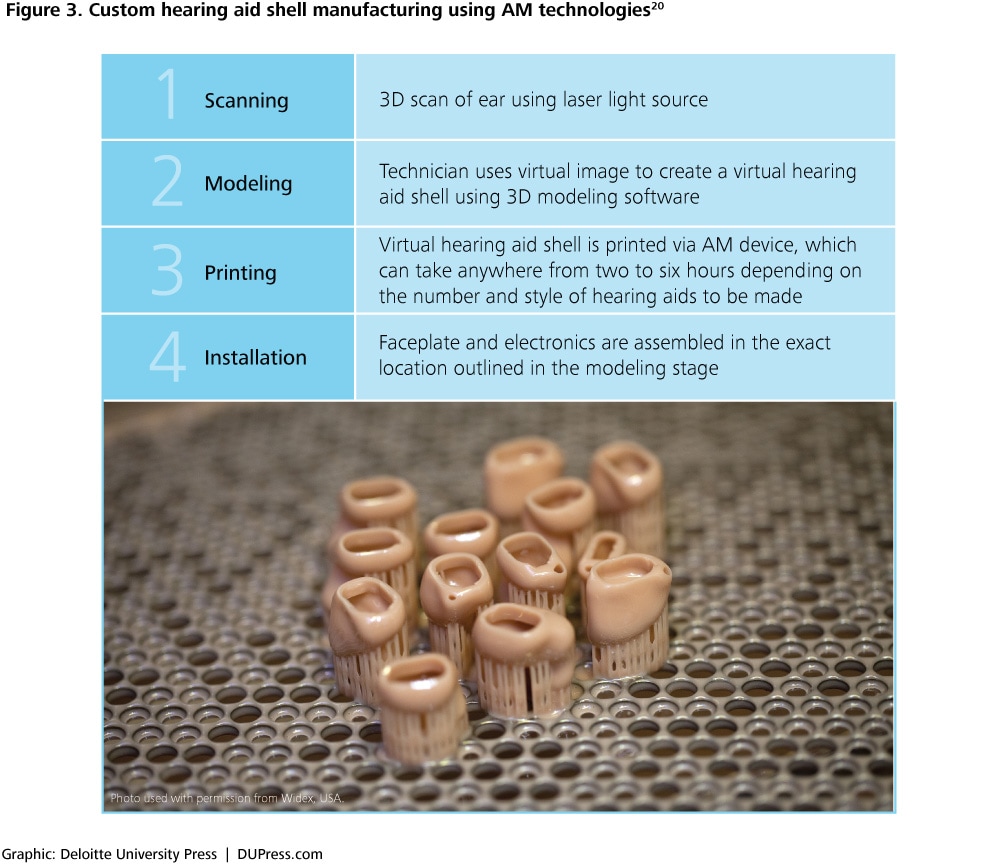
For example, AM has already significantly changed the way in which custom hearing aids are delivered. Traditionally, the process of producing a custom-shelled hearing aid involves the steps outlined in figure 2. The application of AM technology in this manufacturing process has led to changes in its execution. A typical AM-enabled manufacturing process for hearing aids is given in the four steps outlined in figure 3.
See endnote 19
See endnote 20
Relative to conventionally manufactured hearing aids, hearing aids made with digital imaging and AM technology are believed to be more comfortable and reliable. They can also be produced more efficiently, and they can reduce the number of repeat ear impressions needed in order to correct fit issues.21 Siemens, one of the world’s largest manufacturer of hearing aids, is switching completely to using AM to produce them at several of its factories.22 Drivers for the change include AM’s ability to shorten the manufacturing process for customized devices by 50–80 percent, localize the distribution of the end product, and significantly reduce labor costs.23
The market for custom dental crowns provides another example of AM’s impact on supply chains in the medical device segment. In contrast to traditional hand-molding approaches, modern dental technicians can now use scanned data and dental software to design a CAD model of a patient’s crown.24 A key benefit of using AM in the custom crown creation process is increased speed of production. EOS, an AM system manufacturer in the aerospace and medical device printing space, asserts that a dental technician who uses traditional production methods can produce around 20 dental copings per day. In comparison, the use of AM allows for up to 450 crowns and bridges to be created in one 24-hour production run.25 In 2013, dental labs used EOS AM machines to create more than 1.5 million direct metal copings and bridges.26
AM influences inventory levels and distribution
AM is also influencing medical device supply chains through its ability to impact inventory and distribution. A key function of inventory is to buffer against demand uncertainty when product lead times are long. Organizations may pursue the use of AM in their supply chains in order to reduce offshore manufacturing and inventory. AM may also reduce the steps and the number of components required to produce end products, resulting in a lower need to maintain subcomponent inventory. Finally, firms employing AM in their supply chains may be able to stay closer to end-product point of use, developing a leaner, more cost-effective supply chain that relies less on safety stock and requires less of a working capital commitment.
The US military has become a proving ground for many of these concepts. For example, the military has actively explored the use of AM within field surgical settings. Typically, combat support hospitals can perform only a limited set of procedures due to restrictions on the weight and volume of equipment that can be transported to a field location.27 This limitation creates the need for the expensive and time-consuming process of shuttling soldiers and equipment back and forth to military hospitals for certain procedures.
AM is also influencing medical device supply chains through its ability to impact inventory and distribution.
To address this issue, the US military conducted a 90-day evaluation of AM in producing on-demand, remote-site surgical equipment. Standard procedures for making surgical equipment available raised challenges related to time of delivery, quantity, and cost; it was also difficult to match supply with demand on the battlefield using standard procedures.28 In its work with AM, the military demonstrated the feasibility of producing surgical equipment using commercially available AM devices. Electrical power, raw material, and digital design files for each instrument were all that was needed to print instruments on demand. In theory, thousands of different surgical instrument designs, or even customized instruments—stored on digital media or remotely accessed via the Internet—could be available for printing and use in field surgical settings.29Through its experiments, the US military has also demonstrated the ability to use AM for the production of sterile surgical kits.30 This may allow military surgeons to conduct more types of procedures, reduce the inventory required for those procedures, and decrease uncertainty surrounding supply levels on the battlefield.
Outside of military applications, Protaico, a provider of surgical guides in Latin America, has been able to move its production of dental surgical guides in-house by incorporating AM into its supply chain. Facing increased demand, Protaico realized that it needed to achieve faster throughput without compromising standards or significantly increasing the size of its staff or inventory. Whereas prior to its use of AM for in-house manufacturing, Protaico cited a historic need to reject orders or outsource the production process, with AM, the company believes it can now quickly fulfill customer orders.31 Protaico also claims that its investment in AM delivered returns in the form of higher revenues and lower operating costs.32 The firm can now offer customers dental appliances with very short lead times at increasingly competitive prices.33
While many organizations are still assessing the use of AM in their supply chains, in the long run, supply chain evolution may represent a strong growth opportunity for AM as companies look to use AM to decrease unit cost and delivery time as well as to improve product delivery precision.
On path III, companies take advantage of the scope economics offered by AM technologies to achieve new levels of performance or innovation in the products they offer.
Path III: Product evolution
Path III deployments of AM offer companies the opportunity to improve financial performance and revenue growth through the ability to create innovative end products that, in the absence of AM, would be difficult or impossible to manufacture. The ability to combine multiple small parts into a single additively manufactured part, for instance, or to customize components of a product, offers compelling opportunities.
Because AM can improve product fit and alter the end-product build, current applications within the medical device space typically focus on enhanced customization. Examples can be seen in AM applications related to improving visualization (in preparation for surgical procedures) and increasing the clinical efficacy of implants and other devices.
Customization aids visualization
To limit surgical risk, it is vital that surgeons have ample practice, much of which comes from performing trial runs on patient-like models. As surgical procedures grow in complexity, many doctors are turning to AM to help create personalized guides for detailed procedures. Surgical guides help doctors understand key structural attributes of the anatomy that they are likely to encounter during surgery.
Surgical guides created using AM are typically of human organs such as livers and kidneys, body cavities, and bones. For example, kidney or liver models based on CT or MRI scans of a patient can be constructed out of a translucent material composed of acrylic resin.34 These models allow surgeons to see and understand organs’ internal structure, such as the location of a tumor or the path of blood vessels. They can also allow surgeons to plan for different operating approaches prior to surgery to avoid the need to make critical decisions on the spot.
One drawback of many traditionally created surgical guides is that they do not replicate the properties of the live patient precisely enough. This leads to concerns that procedure quality may suffer. Take, for example, the use of surgical training models for the sinus cavity. Because the sinus cavity resides within millimeters of the brain cavity, precision is essential.36 A Loughborough University team aiming to improve surgical training techniques has created AM-produced models that replicate the appearance and physical structure of the human sinus.37 Each individual’s sinus is unique and contains both hard and soft tissue in various configurations; these lifelike training models more accurately depict the human sinus and prepare doctors for the many complexities of clinical scenarios.
The increased verisimilitude of additively manufactured surgical guides provide surgeons with improved training tools and allow them to spend less time in the operating room. Surgery costs today are estimated to be roughly $100 per minute, and the use of AM to create surgical guides can help to reduce the time a patient spends in the operating room as well as speed up the recovery process.38 Additionally, there is hope that improved surgical guides can reduce complications from surgery, as doctors can obtain a detailed knowledge of a patient’s anatomy prior to the procedure. More than 70,000 surgical guide units were produced in 2013.39
Customization aids clinical efficacy
The pursuit of benefits such as lower hospital costs due to less time spent in the operating room, as well as a general trend toward increased end-product customization, has increased the demand for additively manufactured custom implants.40
For example, University of Michigan researchers were able to use AM to create a customized implant for a young boy with a rare disorder that led to frequent lung collapses. Doctors concluded that the patient needed a customized tracheal implant. They used AM to print a customized bio-absorbable splint that was flexible enough to grow with the child. Researchers created a digital model of the splint using a CT scan and then “printed” it out. The implant is expected to remain in place for three years before being absorbed by the body.41 Thanks to the success of the custom-built splint, the boy was taken off of his ventilator just weeks after his surgery.
Other examples illustrate the versatility of AM-driven custom product approaches. For instance, AM technology was used to create a custom titanium jaw replacement for an 83-year-old woman. An advantage of the AM-created implant was to reduce surgery time from an average of 10–20 hours to around 4 hours, since fewer adjustments were required with the AM-manufactured piece.42In this instance, the patient was released from the hospital after only four days, rather than the typical two to four weeks associated with more traditional mandible replacements.43
Demand for low-volume, highly customized products with life-dependent outcomes creates strong conditions for alignment between the medical device space and AM. Although few companies have pursued AM for product evolution in depth, it is anticipated that as the technology continues to establish itself, more companies will see AM’s potential benefits for product development and apply it to future production.
On path IV, companies alter both supply chains and products in pursuit of new business models.
Path IV: Combined supply chain and product evolution
Path IV brings together the supply chain changes of path II with the product impacts of path III. Firms on path IV look to create new value delivery methods to innovate their business models, increase growth opportunities, pursue new markets, and impair competitors’ ability to compete.44 Dominant themes along this path include mass customization, on-site manufacturing of custom devices, and bioprinting.
Mass customization brings lower cost and better fit
Mass customization combines the low unit costs of mass production with the flexibility of individual product customization. The use of AM to mass-customize medical devices offers an example of ways that product evolution and supply chain modification can work in concert to deliver an improved product more efficiently.
A relatively new application of AM in the medical device space is the creation of custom insoles to reduce foot pain and improve posture. Custom insole creator Sols plans to offer a completely customized product using a novel production process.
In traditional custom sole manufacturing, the process generally involves taking casts of the patient’s feet and then sending them to a lab for analysis. The multiple human touchpoints in the traditional process leave room for error. With AM, Sols has replaced the need for these error-prone touchpoints, streamlined the production process, and created a product with unique opportunities for customization.45
Sols’s custom sole creation process starts with using a smartphone app to scan the patient’s foot and upload it to Sols’s customer database.46 After the scan is verified, the image is transferred to an AM device to produce a model that is subsequently used to create a personalized insole. Customers choose a color, and the insole is coated with an antimicrobial topcoat that repels sweat and odor. Sols believes this streamlined production process and high level of customization will allow it to efficiently mass-customize its product for customers when the product launches in 2014.
On-site product customization reduces inventory and improves performance
Using AM in a firm’s supply chain and product development life cycle can allow manufacturers to rely less on standard customization techniques, maintain lower safety stock in inventory, and lower their commitment of working capital. As custom products move to a just-in-time inventory model, manufacturers will spend less money on the overhead required for inventory and warehousing.
For example, surgeons who today use standard “off-the-shelf” knee implants for replacement surgeries must choose from a range of fixed sizes and then make necessary adjustments in the operating room to determine the implant’s final fit.47 Because standard implants are not tailored to each patient’s specific anatomy, surgeons often must compromise on implant fit. This compromise may range from implant overhang (when the implant hangs over the bone) to implant underhang (when the implant is too small, leaving the bone uncovered and misaligned), thus causing residual pain post-surgery.48
In the future, AM may be used to scan a patient and immediately produce a more nearly perfect implant on site, offering doctors the ability to better manage inventory and reduce procedure time. Conformis, a company that focuses on the image-to-implant process, offers a knee replacement system that it believes offers unique advantages not possible with off-the-shelf implants, including a more individualized fit that virtually eliminates the sizing component during procedures while enabling just-in-time delivery of the end product.49 At Conformis, the process starts with a 3D scan of the patient’s knee, which is used to create a model that corrects for imperfections related to bone spurs, cysts, or joint flattening. The design is then produced using AM and delivered on-site to a surgeon just days before surgery, relieving hospitals of the need to keep inventory on the shelf for extended periods of time.
Although Conformis does not currently offer on-site implant printing, its process highlights the opportunity for customized knee implants to be printed on-site or delivered quickly to the site. While advancements in customized, on-site implant production with AM remain limited at this time, many companies are exploring this avenue to realize the potential cost savings available through AM’s inventory management capabilities.
Bioprinting made possible
Every 30 seconds, a patient who could have been saved with tissue replacement dies instead.50 This statistic alone motivates research and investment in bioprinting. Bioprinters construct living human tissue by printing successive layers of human cells. AM using human tissue helps create cell-cultured platforms without the need for biomaterial or other scaffold components that would not have been found in native tissues.51 Advancements in bioprinting portend future development of new products, as well as bioprinted tissues and organs for research. Currently, most bioprinters are in the early stage of development; however, if the promise of these technologies is realized, they may revolutionize the medical industry.52
Several examples illustrate early progress in bioprinting. For instance, Organovo has been able to create living human tissue that successfully mimics the form and function of human organs.53 The company sees its biggest success as being the creation of the first three-dimensional human liver tissues, with human cells arranged in patterns similar to those in a live structure. Organovo’s goal in producing liver tissue is to construct living, multicellular human tissues that can be maintained in a laboratory environment for extended periods of time. The company hopes to one day produce fully functional organs through AM.54 Meanwhile, the current production of additively manufactured liver tissue offers a promising platform for future medical research.
Within the medical device sector, companies aligned to path IV are seeking to transform both their supply chain and their products. Thus, only a few medical device companies are on path IV today. However, as AM continues to grow and develop, companies using AM may see great benefits as costs fall, supply chains become more streamlined, and products become more advanced.
AM’s increasing adoption: Limitations and opportunities
AM offers medical device companies many opportunities to improve performance, drive innovation, and pursue growth. However, certain factors may slow the adoption of AM in this sector, including increased regulation, technology shortcomings, and a shortage of talent with AM printing capabilities.
Over the past five years, the regulatory environment surrounding AM products has evolved.
Increased regulation
Over the past five years, the regulatory environment surrounding AM products has evolved. Controversial applications of AM—such as 3D-printed guns—have increased regulatory scrutiny of AM-created products. The US Congress is in the process of extending a ban on the production of firearms capable of evading metal detectors and X-ray machines, including those made using AM devices. In addition to firearms, AM technology has the ability to manufacture other products that are normally subject to regulatory control. The medical device sector falls into this category.
Within the medical device segment, the regulatory process to approve new device classes and new manufacturing processes is lengthy.55The US Food and Drug Administration (FDA) and other global governing bodies may take 7-10 years to approve a new product and allow it to go to market. Though few regulations specifically targeting AM for medical devices currently exist, regulation by the FDA and other bodies is expected to increase in the coming years.56
Structural strength
The structural strength of materials used in AM also presents an area of ongoing development. Because the AM production process typically occurs “layer by layer,” end products are known to have good strength on the X and Y (length and width) planes but are feared to lack equivalent strength on the Z plane (depth).57 While the layer-by-layer process has proven strong enough for many applications, concern remains that products created with AM could be less structurally sound than those produced with traditional manufacturing.58 Managers should keep watch for developments in this area, as their impact on clinical efficacy may be significant.
Speed and size
Currently, it can take hours to produce even relatively small objects using AM. Although the printing process can be shortened by adjusting product thickness and size, this may decrease the end product’s surface and finish quality. While a few days to print a prototype is an improvement over traditional manufacturing methods, the AM process may need to be accelerated before local practitioners see fit to adopt it. Until then, the ability to fully distribute production may be limited.
Talent shortage
 The rise of AM will drive a greater need for training and development of skill sets specific to AM operation. Skills will be required in the areas of computer-aided design (CAD); building, operating, and maintaining AM machines; raw material development; and supply chain and project management.59 Because AM is a relatively new technology, most of the training around AM operation is currently offered on the job instead of in formal training sessions. As AM continues to proliferate, there will be a greater need for structured, comprehensive training and skills development in this space. Companies should watch for opportunities to encourage the development of medtech-specific AM training opportunities, as well as to pursue access to them.
The rise of AM will drive a greater need for training and development of skill sets specific to AM operation. Skills will be required in the areas of computer-aided design (CAD); building, operating, and maintaining AM machines; raw material development; and supply chain and project management.59 Because AM is a relatively new technology, most of the training around AM operation is currently offered on the job instead of in formal training sessions. As AM continues to proliferate, there will be a greater need for structured, comprehensive training and skills development in this space. Companies should watch for opportunities to encourage the development of medtech-specific AM training opportunities, as well as to pursue access to them.
Looking forward
AM is anticipated to have a significant impact on the medtech industry—in particular, the medical device sector. Adoption of AM is likely to increase as more firms come to appreciate its potential benefits across their supply chains and products.
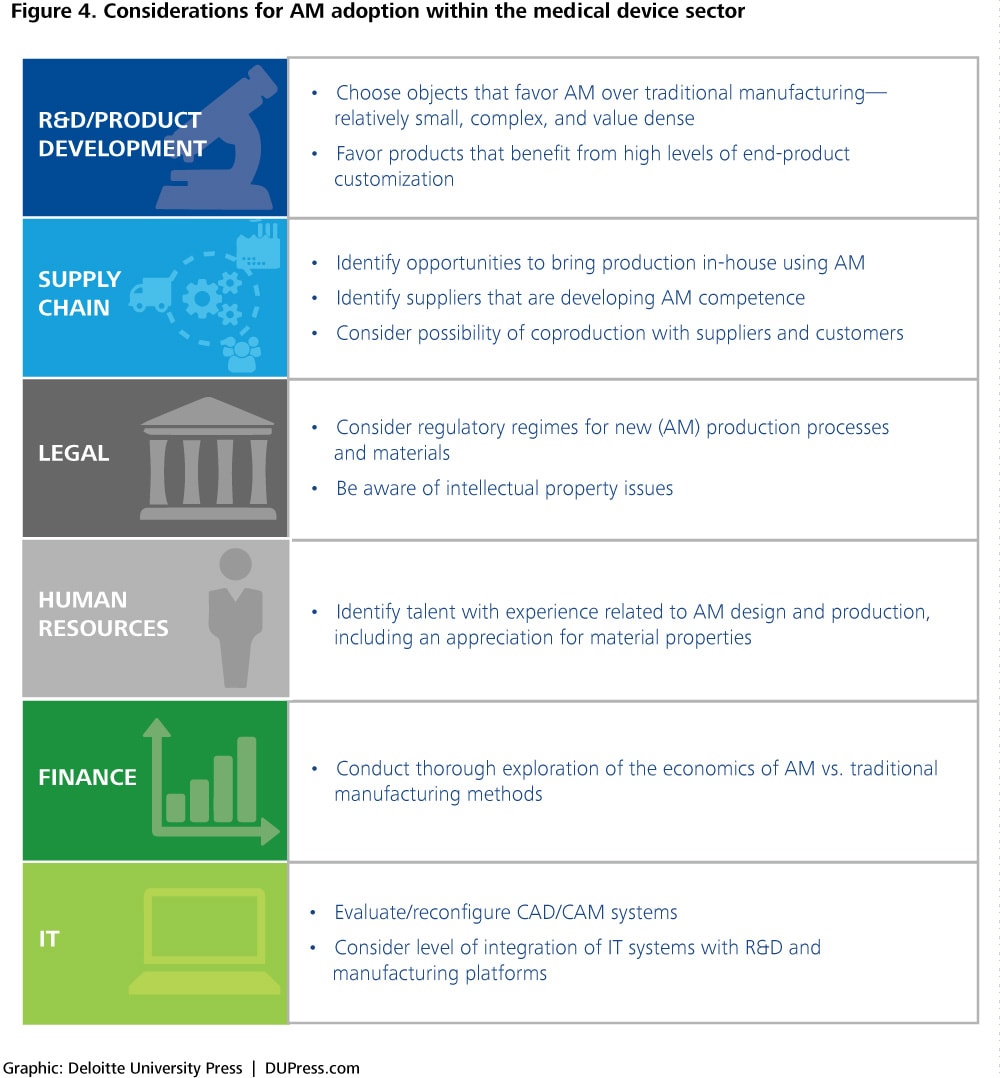
The decision of which tactical path to follow in adopting AM depends largely on a company’s value drivers and strategic goals. Figure 4 summarizes key issues for leaders to consider when pursuing AM adoption.
Medical device companies that want to improve their competitiveness while incurring relatively little risk might choose path I (stasis). This path can allow them to address both the profitability and speed of their current operations. As discussed, most companies currently follow this path by adopting AM for rapid prototyping and modeling.
Companies that are more concerned with the competitiveness of their supply chains may want to consider path II (supply chain evolution). Along this path, firms focus on overall performance improvement rather than product innovation. Although the application of AM in the supply chain is still evolving, some companies that have incorporated AM into their supply chains have seen benefits from greater supply chain and inventory distribution efficiencies.
Companies might also choose path III (product evolution), focusing on breaking trade-offs imposed by traditional manufacturing methods and pursuing innovative changes to products. Companies considering this path may wish to explore opportunities related to mass customization and bioprinting. As with path II, this approach to AM, while still in its infancy, is anticipated to become more widespread.
Companies that wish to advance along path IV (combined supply chain and product evolution) must overcome a number of hurdles. Although the challenges outlined in this report exist to some extent along all paths of AM adoption, they may matter most to those that hope to use AM to achieve true business model innovation. While path IV has attractive features, it is also the riskiest, and hence represents the least common path for companies within the medical device sector.
Industry participants should closely monitor the evolution of business models based on the deployment of AM technologies. The possibility of rapid change in key markets—as has occurred with devices such as hearing aids and dental crowns—is real. Additionally, medical device firms should begin to develop a long-term strategy surrounding AM. Although not all companies will want to make substantial strategic investments at this time, leaders should consider devoting attention to where the industry and the individual segments they serve are moving. In some cases, a “wait and see” strategy may be appropriate.
As the advantages AM can offer over traditional supply chain and production methods become more evident, companies in the medical device sector will likely become more open to the use of AM. The constant need to innovate and grow will give medical device companies the impetus to continue to explore and adopt AM technologies.
Deloitte Consulting LLP’s services to medical technology companies combine life sciences/health care industry knowledge with supply chain and manufacturing capabilities to help organizations apply advanced manufacturing technologies to support their businesses’ performance, innovation, and growth. Our insights into additive manufacturing allow us to help organizations reassess their people, process, technology, and innovation strategies in light of this emerging set of technologies. Contact any of the authors for more information or read about our alliance with 3D Systems and our 3D Printing Discovery Center on www.deloitte.com.
© 2021. See Terms of Use for more information.