Operate for Life Sciences has been saved

Solution
Operate for Life Sciences
Expertise you can trust for the management of your data
Our Operate services offer specialised operational support to the life sciences industry for data and label management, utilising automations and tooling for optimal efficiency and pairing that with in depth industry knowledge to work collaboratively with our clients as an integral part of their team.
Your Challenge
With the move from unstructured to structured data submissons, the challenge of collecting, remediating and maintaining, high-quality data is uppermost for Regulatory Affairs teams. The successful management of that data is labour and time intensive, requiring expertise to ensure the good data quality critical to avoid delays to submissions and approvals.
Why Iperion – a Deloitte business
Our Operate team includes regulatory experts to support you in all your data needs, whether as a one-time activity or as part of a continuous improvement or management program. Services for data remediation, regulatory data management and labeling are developed with a data driven approach and collaborating with your teams to optimise your data insights, offering you agility and dynamic control, with confidence.
Our Solution
Our Operate services bring together our experienced specialists, proven processes, and market leading data expertise for the provision of data collection, data remediation, and data and labeling management services. When clients Operate with Deloitte, they embed experts into their teams, freeing them and their people to focus on regulatory strategy and getting drugs to patients.
We provide a range of services, tailored to our clients requirements, enabling successful digital transformation for regulatory in life sciences.
- Data collection, remediation, and data entry
- Data Management
- Label Management
Data Remediation
Deloitte’s data remediation tooling efficiently creates structured data from (unstructured) information. Using artificial intelligence powered technology, we can extract all relevant data elements from your regulatory documents. Our IDMP offering of data remediation as a service is designed with a 4-step approach including extraction of the data elements, remediation to align the data with IDMP standards and verification performed by IDMP SMEs. The output is aggregated in the format you require to import into your RIMS.
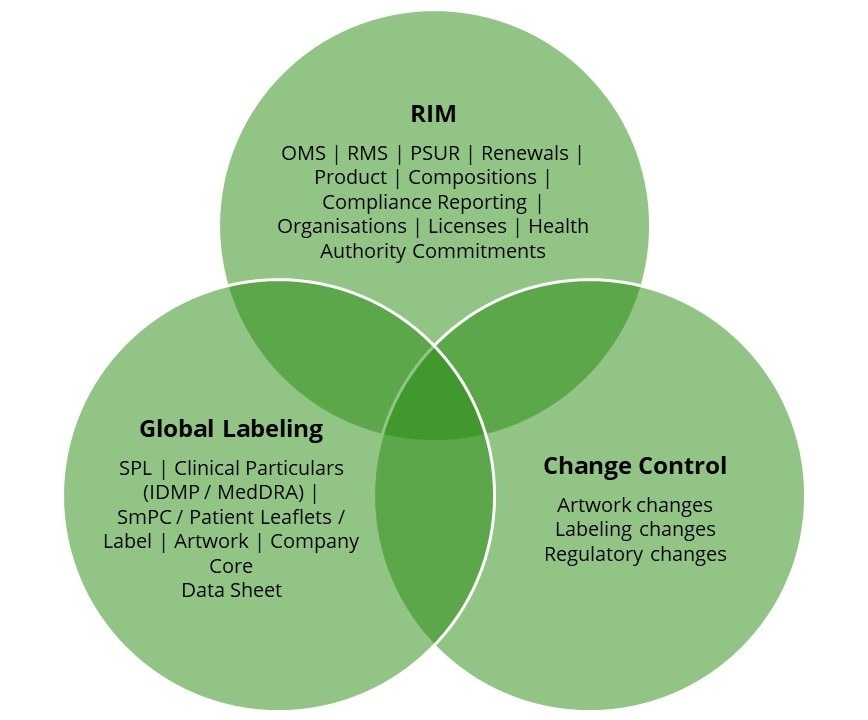
Data Management
Achieving a high level of data quality and trustworthiness for your (regulatory) product information is essential for any organisation. It requires subject matter expertise, experience and knowledge. Our team can assist you with incorporating the latest data requirements (e.g. PLM, ISO IDMP, PQ-CMC, TOM). We provide dedicated resources with the right capabilities and knowledge to enable you to focus on strategy, development, innovation, while we guarantee data quality.
As part of our service, we re-engineer your data management processes to improve efficiency and maximise effectiveness, while simultaneously increasing the quality of your data.
We supply regulatory data services that keep you in control and in compliance of your products, in combination with our consultancy services.
Our team can support you with the management of:
- RIM
- XEVMPD
- PLM
- SPOR
- ISO IDMP
Label management
Authoring and maintaining the label documentation for a medicinal product is one of the most important elements of the authorisation processes. Getting this right is critical to maintain compliance and to ensure that patients and healthcare providers always have the latest information at hand.
The combination of Deloitte’s advisory expertise paired with the Operate team’s provision of Labeling as a Service sets our clients up for success.
Our experts will work in collaboration with your teams to establish a clear label management process; label and data quality control, proofreading (etc) that fits with your established business model and existing technical solutions, supported by our data experts for label management.
Our team can support you with:
- Technical authoring
- Document migration
- Document Quality Control and proof reading
Recommendations
Iperion life sciences consultancy becomes part of Deloitte
Our commitment to promote data standardisation for better patient outcomes



