Tackling digital transformation How biopharma companies can realize the power of digital and prepare for a new reality
17 minute read
17 July 2019
Digital technologies, which are driving massive transformation in health care, can help biopharma companies innovate to develop products and services, engage better with consumers, and execute operations more effectively.
Executive summary
The explosion of digital diagnostics and therapeutics, increasing patient-centeredness, interoperable data, and increased regulatory collaboration are all signs of the tremendous changes that digital technologies are driving in the health care industry.
How can biopharma prepare for this new reality? How should biopharma companies transform their operating and business models to compete?
Learn more
Read more from the Life sciences collection
Find out more about digital transformation in life sciences
Explore Deloitte's podcast series on digital transformation
Subscribe to receive related content
Download the Deloitte Insights app
Participants in our crowdsourcing research believe that digital transformation can enable the innovation of new products and services, increase customer engagement, and improve execution of operations:
In research and development (R&D), digital transformation has the potential to drastically improve productivity by applying artificial intelligence (AI) and computational biology to drug discovery and development and by making clinical trials much more efficient.
In commercial, more targeted patient engagement and the application of behavioral science principles could lead to better patient outcomes. Persona-based marketing to health care providers could lead to greater market awareness and action.
In supply chain, digital supply networks can result in much greater product visibility, traceability, and inventory control.
How can companies leverage the promise of digital? Each biopharma company should chart its own course. The first step is to set an ambition and execute against it. Our research suggests that companies often need five key competencies to get to the future state: advancing scientific knowledge through access to new data sources, applying the principles of behavioral science to improve patient outcomes, establishing an enterprise architecture structure, developing predictive analytics and AI capabilities, and enabling cultural change.
Setting the vision: Early signals in the market point to large-scale disruption in the health care industry
Digital technologies are enabling new diagnostics and therapeutics. Data is the new health care currency. Massive amounts of data are being generated from patients using consumer sensors and monitors, at-home diagnostics, and digital therapeutics. For instance:
Deloitte’s 2018 consumer survey results show 42 percent of consumers use technologies such as wearables and sensors.1 These devices continuously gather data and their capabilities are increasing: The most recent version of the Apple Watch® wrist wearable device incorporates an electrocardiogram functionality to help detect arrhythmias in users.2 This data could provide real-time insights into users’ health and response to treatment.
Direct-to-consumer genetic tests are becoming increasingly popular, making genomic data available for research and personalized medicine. Digital therapeutics are emerging as alternatives or supplements to drug interventions in disease areas ranging from asthma to Alzheimer’s to chronic obstructive pulmonary disease, and diabetes, to name a few.
The patient is at the center. Digital technologies are enabling patients to control their health care information and partner in their care decisions.
Organizations such as Ciitizen and Apple (through its health records API) are empowering patients to organize, control, and share their health care information.3 Increased ownership can enable patients to share this data with parties they trust, and support the creation of connected devices, apps, and other services to better support patients on their care journey.
Deloitte’s 2018 consumer survey shows that 60 percent of consumers are willing to share personal health records with their doctor.
The market is making advances with interoperable data. With industry groups, federal agencies, and technology companies enabling data interoperability, patient data can more easily be used for holistic and longitudinal analysis of health outcomes.
The Fast Healthcare Interoperability Resources (FHIR) is creating new data standards to promote interoperability across the health ecosystem. The Office of the National Coordinator for Health Information Technology is focused on using standards such as FHIR, encouraging standards for API use, aligning incentives, clarifying privacy and security requirements, and coordinating among stakeholders.4
The National Institute of Health Data Commons is being developed to accelerate new biomedical discoveries by testing a new platform where investigators can share, access, and interact with data and software.5
Evolving technologies, including blockchain,6 can facilitate interoperability while ensuring data security.
Regulatory collaboration is on the rise. The US Food and Drug Administration’s (FDA) Digital Health Innovation Action Plan included a pilot Digital Health Precertification Program which resulted in a draft regulatory framework to test new approaches to the oversight of digital health tools.7 This is a major step forward for the industry and has already resulted in the FDA’s clearance of digital therapeutics by companies such as Pear Therapeutics and Omada Health.
Given these signals, we foresee a period of rapid transformation for the health care industry and biopharma sector. Incumbents should proactively leverage these disruptive dynamics. Lack of transformational vision could create opportunity for startups and new entrants to disrupt the industry from outside.
We conducted a crowdsourcing simulation with 38 industry leaders to learn how digital transformation could enable biopharma companies to move toward new business and operating models (see “Research methodology”).
Research methodology
In January 2019, Deloitte led a four-day online crowdsourcing simulation involving 38 industry leaders. The crowd included executives from biopharma manufacturing, R&D, marketing, strategy, and innovation functions; technology companies and startups; academics; thought leaders; venture capitalists; physicians; and payers.
We asked the crowd to assume that data interoperability will become a reality, scientific and technological advances will proceed at an exponential pace, and regulators will keep up with the pace of change. The crowd actively deliberated on how biopharma companies and their functions could evolve in the context of digital technology disruption.
Imagining the digital transformation of biopharma companies
The crowd overwhelmingly saw technology as a driving force in the transformation of biopharma companies. The availability of rich datasets and analytics could mean a shift from traditional drug development using large-scale clinical trials to the storage and computation of big datasets that allow companies to develop highly effective personalized therapies. Applying cognitive technology to aggregated data, companies could find ways to automate R&D processes and reduce development costs. Always-on sensors capturing patient data could provide better outcomes data, and behavioral “nudges” could improve patient adherence to treatments or lifestyle.
A better understanding of patient-specific disease characteristics could enable more effective, targeted therapies. The exponential change in biomedical research and consumer pressures are also likely to shift the treatment of today’s most common diseases from symptom management and disease modification to a focus on prevention and cures.
Digital transformation is the next step in the evolution of biopharma companies. For successful change, companies should innovate new products and services, engage customers effectively, and execute processes efficiently (see figure 1).
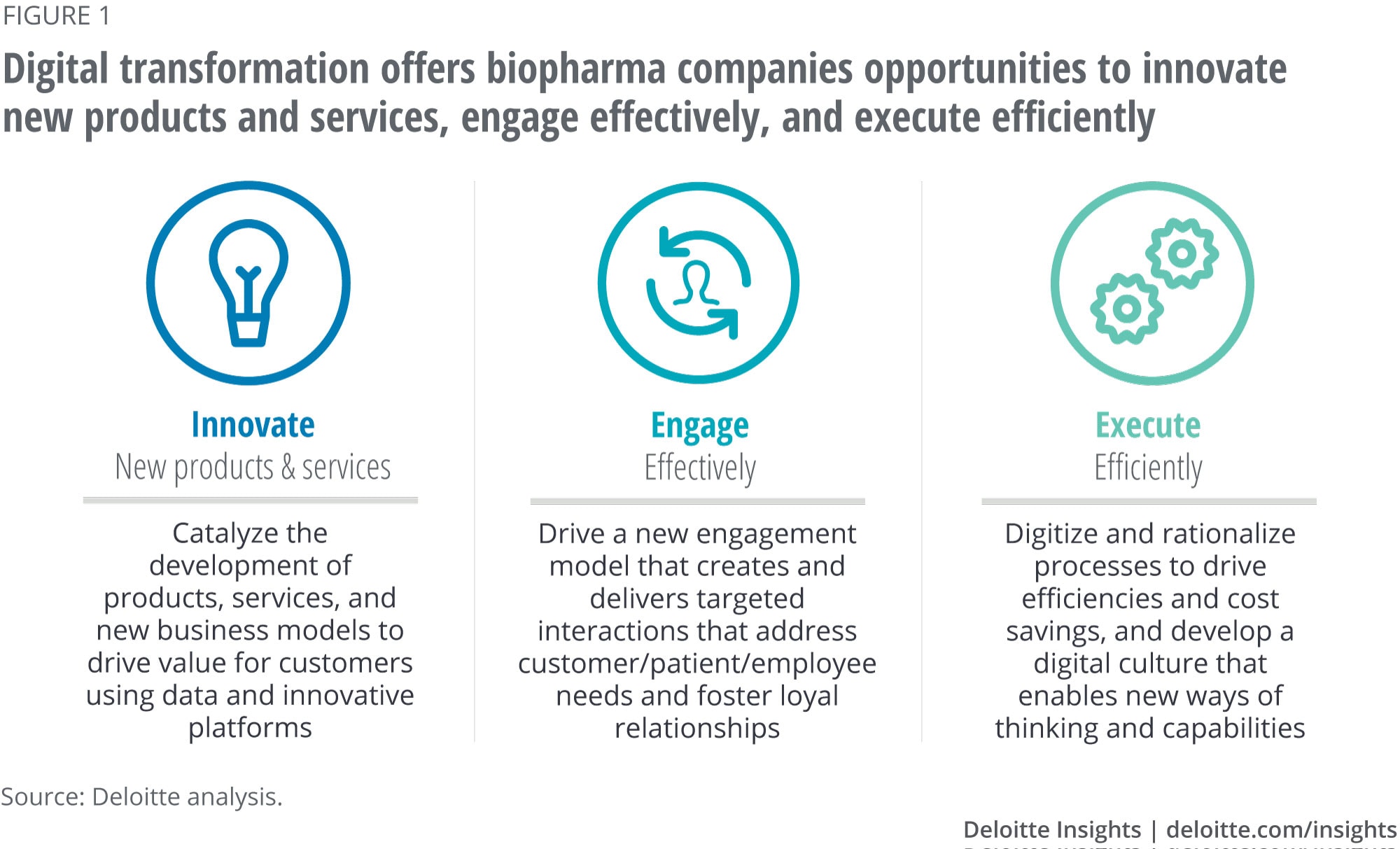
Today, the biopharma industry is primarily leveraging digital technologies to modernize existing legacy processes, but a big opportunity lies in exploring new ways of doing business digitally. For instance, the crowd agreed that digital transformation opens up new avenues to:
Innovate. Several emerging technologies will likely be innovation springboards for biopharma companies: Cell and gene therapy (including viral vectors), nanotechnology, 3D printing, implantable drugs combined with microchips, and other novel drug delivery technologies all have the potential to drastically change the types of treatments biopharma companies offer. From hyperpersonalization of therapies to greater efficacy of existing API and supply chain disruption, the crowd anticipates these technologies will have a significant impact on the industry. Furthermore, a better understanding of disease biology, the patient journey, and environmental/lifestyle studies can enable biopharma companies to prevent or treat a wide range of diseases with customized products, services, and solutions.
Engage. The crowd agreed that companies are already moving in the direction of developing hyperpersonalized offerings. Today, biopharma companies are already beginning to provide customized disease management programs. However, these offerings are likely to quickly evolve to emphasize prevention and behavioral modification. These offerings could include an integrated set of products and services including diagnostics, real-time monitoring apps, drug product/dosing recommendations, and other lifestyle support/behavioral nudging to prevent chronic diseases. AI can be leveraged to assess outcomes data and granular patient attributes (body type, metabolism, medical history), which can help identify different patient types that would respond best to a treatment option.
Execute. The crowd discussed how digital transformation could help companies improve their processes and productivity. Specifically, biopharma companies should be able to reduce R&D costs, better target marketing spending, and optimize manufacturing and supply chains. This increased efficiency can enable the delivery of personalized products and solutions that are affordable despite the health care system being increasingly cost-constrained.
How will biopharma functions transform?
The crowd discussed how functions within biopharma companies would each need to transform. A few early movers are starting to take first steps to enable this transformation.
Research and development
A major point the crowd agreed on was that R&D should change its approach from a “solution seeking a problem” to better understanding the patient condition and then developing a way to prevent, treat, or cure complex diseases—a drug, a supplement, a digital therapeutic, or a combination of these. This will likely require a more personalized approach and an understanding that what works for one patient may not work for another.
The crowd agreed that AI applied to diagnostics and other novel data sources can have the biggest impact on biopharma success. To achieve this, R&D functions should collect and analyze multiple datasets (real-world data, genomics, clinical, and claims) to map the whole patient journey (disease onset, progression, and environmental influences). Predictive modelling and in-silico testing can improve target identification, assess safety and efficacy, and reduce the number of failed drugs. Many early movers are scratching the surface by experimenting with AI to expedite target compound identification and testing (see case study 1).
 Case study 1
Case study 1
Early movers: AI platforms for lead molecule screening and selection
Some companies are already collaborating with AI companies such as Deep Genomics, Atomwise, Exscientia, and others to make discovery and preclinical testing activities nimbler, with higher throughput.
For example, Exscientia, through its AI platform, is automating the analysis of existing discovery data (including journals, patents, databases) for the identification of novel compounds for synthesis. The platform preassesses each of the identified compounds for predicted potency, selectivity, absorption, distribution, metabolism, excretion, and other key criteria. The platform can cut down the time needed to identify drug candidates to roughly one-fourth of the time taken by traditional methods.
Sanofi, Sunovion Pharmaceuticals, and GlaxoSmithKline are each trying to use Exscientia’s machine learning platform to find novel lead molecules for disease-related targets across therapy areas.
Furthermore, “organ on a chip,” 3D cell cultures, and other cell models that replicate human biology in in-vitro testing systems can reduce the need to conduct animal testing and may begin to eliminate Phase 1 human studies (see case study 2).
Shifting from randomized placebo-controlled trials to the wider use of computational models, simulations, and synthetic trials could speed up the time it takes for products to reach the market. Further, digital technologies can create efficiencies in the clinical research process. Our research on the digital future of clinical development captures how digital technologies can automate several labor-intensive processes including patient screening, recruitment data capture, and documentation.8
 Case study 2
Case study 2
Early movers: Testing the next generation “lab rat”
Early movers Roche and Takeda are collaborating with Emulate to incorporate its “Organ-on-a-Chip” technology into their R&D labs. “Organ-on-a-Chip” is a microfluidic cell culture chip that simulates the activities, mechanics, and physiological response of tissues, organs, and organ systems.
Roche is using Emulate’s “Lung-Chip” and “Brain-Chip” to personalize safety testing by using chips with patient-derived cells to test how a patient or patient group might respond to a drug.9 Takeda is using Emulate’s “Intestine-Chip” to replicate the microenvironment in the intestinal epithelium (cellular structures, cell interactions, and microbiota). This can help in the study of intestinal disease mechanisms and testing of potential new treatments for gastrointestinal disorders such as inflammatory bowel disease.10
Commercialization
In the era of digital transformation, targeted and personalized engagement can be critical to commercial success. However, the crowd disagreed on who should be the main target of these individualized marketing campaigns. Some believed that physicians will continue to be the main decision-makers. Others said that tomorrow’s commercial teams will focus on engaging patients and their caregivers. Further, other ancillary providers such as nutritionists, acupuncturists, and physical therapists are likely to become more influential in the patient journey.
The crowd agreed that messages should be sent at the right time and include customized clinical information, education materials, and interventional and wellness recommendations. The crowd also discussed how direct-to-consumer interaction could require biopharma to behave more like today’s consumer brands (Netflix, Amazon), analyzing and anticipating consumer needs to deliver personalized solutions.
Improved market success and patient outcomes will likely depend on the ability to influence patient lifestyle behavior. Early attempts are already under way to use AI for precision engagement by tailoring behavioral nudges to an individual’s personality, motivations, care journeys, and engagement challenges (see case study 3). Virtual reality apps could provide a visceral experience of disease progression, treatment options, and lifestyle changes to help support patient engagement and brand building.
 Case study 3
Case study 3
Early mover: Identifying precision nudges for diabetes care11
Clinicas Del Azucar, a diabetes care clinic in Mexico, has been using several behavioral nudges (for example, text reminders to walk, exercise, and skip foods with high sugar content) to help its patients maintain optimal glucose levels. The clinic has worked with Deloitte to establish the “Behavior Change Learning Lab” to measure the impact of behavioral nudges on different care goals. These include clinic membership renewals, attendance at three-month appointments, and healthy behaviors and habits.
Analyzing data from Clinicas Del Azucar, the lab is creating predictive models to identify behavioral interventions that may have the most influence on specific patient or population groups. This in turn could help build precision engagement plans for future patients.
Customized health care practitioner (HCP) engagement could help ensure that the right HCPs get the right content at the right time. Some companies have already begun providing HCPs with access to customized content (see case study 4). The crowd expects this to continue. AI can help stratify HCPs into various “personas” based on their habits, preferences, and receptivity to marketing messages. This can help craft and deliver messages tailored to physician needs (continuing education, goals to become key opinion leaders, augmenting sales efforts). Biopharma companies could also utilize alternate/virtual reality to educate physicians on mechanisms of action, drug interactions, and patient responses to cutting-edge therapies.
Evidence generation to establish proof of value will likely become critical to support reimbursement models for new curative therapies and services. The crowd pointed out that real-world evidence (RWE) will likely be foundational to how products and offerings will be paid for. RWE should be able to substantiate which products work for which populations and enable emerging payment models such as value-based contracting. Value-based contracting parameters are also likely to expand to include metrics on the impact of an intervention on patient behavior (adherence to diets, exercise regimens) and even the ability to delay disease onset/progression. The crowd highlighted an example of an experimental payment model for an app-based intervention in Europe. The biopharma company is paid a small fee when the patient initiates the app-based treatment, coupled with bonuses for milestone outcomes. These include reaching/maintaining target levels of disease-specific variables at three-month, six-month, and one-year intervals without any complications. The model ensures the pharma company only gets paid when the app is used as intended and rewarded for patients initiating and continuing the use of the app.
 Case study 4
Case study 4
Early mover: Customizing content for health care practitioners12
A large global biopharma company has built a self-service portal for over 30,000 HCPs across Europe to access marketing and sales content across a variety of mediums. The content shared with each HCP varies depending on their previous viewing habits. This can enable customized targeting based on individual HCP preferences, helping improve the effectiveness of their marketing approach.13
Building trust and transparency
Biopharma companies should gain patients’ trust for them to be willing to share data. This may require ecosystem partnerships with patient advocacy groups, providers, or other groups that patients already trust. Further, biopharma companies should be clear and transparent about how they will use the data, who will have access to it (especially third parties), follow rules and regulations such as the Health Insurance Portability and Accountability Act in the United States and the General Data Protection Regulation in the European Union, and understand that any missteps will likely set back data-sharing goals.
Connected ecosystems will be required to drive secure data exchange between industry stakeholders. Collaborating with neutral third parties and technology companies could help build the infrastructure to notify or seek permission from patients to use their data. Working with industry associations or regulators could help create governance frameworks that could serve as the cornerstone for transparent and secure patient data exchange.
Manufacturing and supply chain
The industry is likely to shift away from linear supply chains to open, dynamic, and interconnected digital supply networks (DSNs). DSNs leverage a variety of digital technologies such as sensors, robotic process automation (RPA), blockchain, and advanced analytics to enable greater product visibility, traceability, and inventory control. A few companies are already seeing benefits from data-driven supply chains (see case study 5).
 Case study 5
Case study 5
Early mover: Data-driven supply chain management for improved inventory control14
A life sciences company developed an interconnected and data-driven supply chain to provide real-time operational visibility and insights. Using a control tower, the company has brought together data from all supply chain functions to create end-to-end product visibility. The company can rapidly assess changing conditions during transit, predict risks before they become issues, and take action. The system has also enabled more informed economic decisions about shipping and better inventory management.
Cost savings and efficiency gains include a 5–8 percent decrease of expedited shipments and a 2–4 percent reduction in backorders.15
Greater product visibility could help monitor demand-supply patterns to proactively suggest product replenishment to customers. Analysis of data from sensors embedded in machinery and using RPA to order parts and schedule maintenance calls could help reduce machine down time. Furthermore, machine learning applied to production data to build compliance and quality risk models could help predict failures and compliance issues, while RPA and natural language processing could automate compliance report creation and filing.
Manufacturing and shipping new products—specifically cell and gene therapies—will likely require a different set of capabilities than traditional molecules. These capabilities should include advanced analytics and workflow automation to seamlessly coordinate patient cell collection, manufacturing capacities, cold chain logistics, and delivery of the viable product to the transfusion center.16
The crowd also suggested that 3D printing could become an increasingly important capability in the biopharma supply chain as the industry moves toward greater personalization of therapies. Many established small molecule therapies could be 3D printed, creating flexibility to tailor dosage and combine drugs into easier-to-take pill regimens. 3D-printed drugs may someday be manufactured at the pharmacy counter, fundamentally changing the types of products biopharma companies distribute to wholesalers and pharmacies.
The time is now
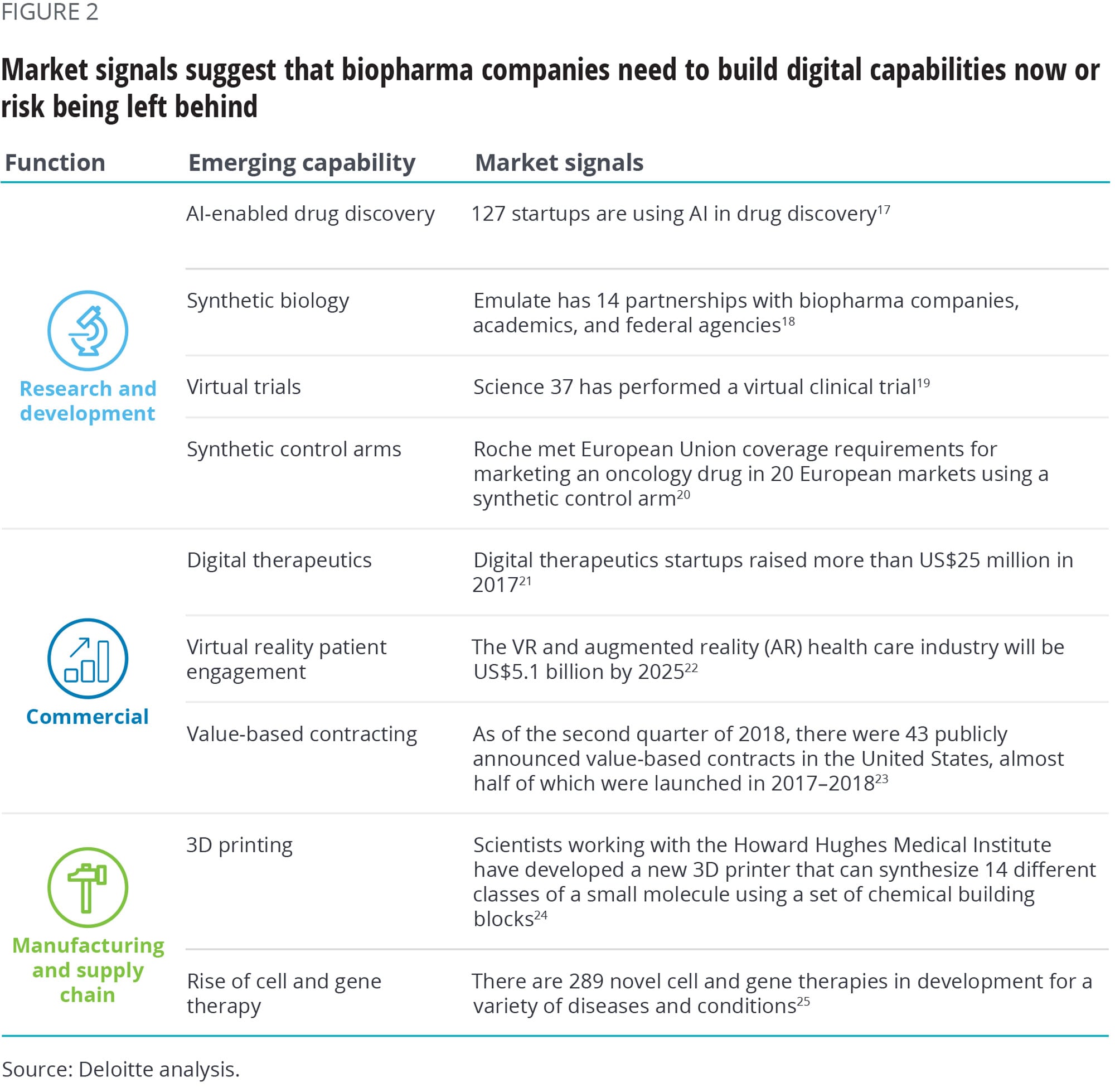
Some early movers are already building the capabilities we describe above. Ultimately, companies that do not build or partner for these capabilities are likely to be disrupted by those that do. Figure 2 summarizes some of the current market activity.
What is required to become a digital biopharma company?
Becoming a digital biopharma company is an ambitious undertaking, and leaders can pursue different paths to get there. A first step is to define a specific vision for the company. In a recent survey conducted by Deloitte and MIT Sloan Management Review, though over half of the biopharma company respondents said that digital investment was a priority for their leaders, they would like leaders to provide a clearer vision and purpose for their organization’s digital investments.26 Leaders can establish a vision by pursuing a top-down approach (looking corporatewide), a bottom-up one (focusing on specific use cases or domains), or a horizontal one (focusing on a key business problem across the life cycle).
No matter what the ambition, there are five key competencies that can be prerequisites to enabling digital transformation.
 Drive scientific breakthroughs through data and data partnerships
Drive scientific breakthroughs through data and data partnerships
Advancing scientific expertise and understanding the causes and modifiers of complex diseases will likely require access to disparate and new data sources. Biopharma companies should leverage ecosystem partners to continue to access, integrate, and analyze this external data. Partnering with or acquiring diagnostic or data companies to access genomic, microbiome, and other personalized patient data can help better understand patient disease characteristics and the patient journey. Companies may also want to increase their presence in innovation hubs to access both scientific and technology talent at academic medical centers as well as emerging startups. Corporate venture capital groups may want to expand their scope to look beyond drug assets–focused companies to search for sources of meaningful data and digital talent.
 Apply the principles of behavioral science to improve patient outcomes
Apply the principles of behavioral science to improve patient outcomes
In the future, meaningful engagement with patients will likely require a deeper understanding of behavioral science, including what motivates individuals to modify behavior in a way that could lead to better outcomes (for example, increasing drug adherence or making lifestyle modifications). Companies should consider embedding nudges or gamification within digital therapeutics or disease management programs that could support patients in improving their overall health. This can become especially important as the market places greater emphasis on wellness and preventative treatments and disease interception before progression.
 Establish an enterprise architecture structure
Establish an enterprise architecture structure
A well-defined enterprise architecture structure and capability can be a critical success factor in developing an efficient technology portfolio that supports scalable growth, speed-to-market, lower total cost of ownership, and increased flexibility. Technology investment should be based on business strategy. Technology blueprints should include a multiyear technology implementation road map that enables enterprise interoperability. The platform will likely be critical to finding, organizing, and analyzing existing internal and external datasets.
 Develop predictive analytics and AI capabilities
Develop predictive analytics and AI capabilities
The crowd identified artificial intelligence as a critical capability of the digitally transformed biopharma company. In R&D, machine-learning computational biology could help identify new drug targets or understand the potential effects of drugs on the human body prior to entering clinical trials. This type of capability could be built in-house or accessed through partnerships with companies such as Deep Genomics, a company that focuses on the intersection of genomics, drug development, and AI.27
Post launch, biopharma companies may also need interoperable health data to track patients remotely, engage, and intervene with personalized treatment options at the right time. This can require the ability to integrate and analyze patient data in real time and determine if/when an intervention is needed. This may require building teams with in-house expertise to collect, manage, assess, and use data to develop predictive modelling capabilities.
 Enable a cultural change: Shift from “doing digital” to “being digital”
Enable a cultural change: Shift from “doing digital” to “being digital”
Core to a successful digital transformation is often a cultural and mindset change such that companies begin to consider themselves not just a science company, but a digital company. Results from the Deloitte and MIT SMR cross-industry survey on digital transformation found that biopharma lags behind other industries in terms of digital transformation.28
Biopharma companies can use Digital DNA, a set of 23 traits identified through research, to guide the tactical actions that are needed to bring about transformation.29 These include democratizing information, failing forward and learning faster, and becoming nimbler.
Further, biopharma companies should hire and empower new leaders who can bring new perspectives, digital experience, and technical skills. These leaders may come from within or outside of the industry and should create a culture that rewards innovation while maintaining focus on operational excellence.
Learn more about biopharma and life sciences
-
Life Sciences Collection
-
Forces of change in health care Article5 years ago
-
Showing cybersecurity's value to health care leadership Article5 years ago
-
Survey finds biopharma companies lag in digital transformation Article6 years ago