
Digital health technology Global case studies of health care transformation
37 minute read
31 October 2019
Around the world, countries and health care organizations are making progress in using health information technology to improve outcomes and access, paving the way for the future of health.
Executive summary
It might seem that health care systems and challenges are local and unique, but there are more similarities than differences.1 Throughout the world, health care systems struggle with affordability, inequitable health care access, uneven outcomes, and increasing demand for services from growing populations with longer life spans.
Learn more
Explore the Health care collection
In a hurry? Read a brief version from Deloitte Review, issue 27
Download the issue
Learn about Deloitte’s services
Go straight to smart. Get the Deloitte Insights app
Health information and digital technologies can help meet these challenges, support population health goals, improve consumer experience, and drive insights into health conditions. We found examples of public and private programs with demonstrated results that offer creative approaches to common problems or lessons for others. In doing so, we begin to see some of the possible features and capabilities for a health system of the future.
Our research found support for Deloitte’s view of the future of health,2 that health care of the future will be different from today in three distinct ways:
- Focus on prevention and well-being. With improvements in early diagnosis, technologies to fix health care problems even before they become problems, and the application of behavioral economics to motivate engagement, care will shift to prevention and well-being. We will move away from the concept of “health care.” Instead, health will be seen as a broader notion that incorporates related concepts of mental, financial, and spiritual well-being. Furthermore, care will be organized around consumers’ needs (as opposed to health care organizations’ needs) and most care delivery will take place outside of health care facilities, in people’s homes, work, school, and in the community.
- This theme is particularly prominent in case studies from India (ReMiND project) and Chile (AccuHealth). A basic mHealth application has elevated community health worker performance and contributed to significant improvements in maternal and infant health outcomes in India’s rural communities. And a combination of remote monitoring, artificial intelligence (AI), behavioral nudging, and nutritional and psychological support has improved quality of life for people with chronic conditions in Chile.
- Interoperable data and platforms. This will give patients, clinicians, and caregivers real-time insights to help with decisions about prevention, diagnosis, and treatment. Decision-support tools will improve care efficiency and effectiveness: Technology will relieve clinicians of most administrative tasks, aid with diagnosis and treatment, and provide additional safety checks.
- We have included five promising examples of data and platforms. National health information technology (HIT) platforms in Estonia, the Netherlands, and Australia have laid the foundation for patient control over their health information and for interoperable and secure data exchange among providers. Two examples hospital systems, from the United States (Geisinger) and Israel (Sheba), boast large improvements in quality and efficiency through traditional analytics and AI.
- Consumer engagement and empowerment. We expect a shift in consumer attitudes and behaviors toward greater engagement in one’s health. With full visibility into and control over their health information, consumers will be able to perform many activities that today require a clinician’s involvement. And technological tools tailored to consumers’ health goals, life stages, and lifestyles will help them look after themselves and their families.
- Three case studies showcase the potential of consumer engagement in improving outcomes. The CANImmunize app enables Canadian consumers to manage vaccination schedules for their families and navigate complex vaccination requirements across provinces. Israel’s emergency response service (MDA) has built a suite of apps to match a user in an emergency with emergency response teams, trained volunteers able to reach the scene before the ambulance, and with other people who carry emergency medicines the consumer in emergency may need. And gamified e-therapy app (SPARX) helps improve emotional resilience among New Zealand youths with depression by teaching them how to apply learnings from the game to real-life situations.
We hope the case studies in this report inspire readers to think about what future digitally enabled health care may look like, how organizations should prepare, and the role they can play in this future.
Introduction
Our goal for this research was to create a collection of curated case studies from around the world to:
- Identify successful uses of health data and information technology in furthering the goals of population health, focusing on achievements that could not have been possible with older technologies
- Examine different approaches to solving common challenges and draw useful lessons
The assembled case studies are not a comprehensive representation of innovative uses of HIT or countries. They are more of an illustration of how some of the future of health concepts are already here. Figure 1 offers a geographic snapshot of the featured case studies.
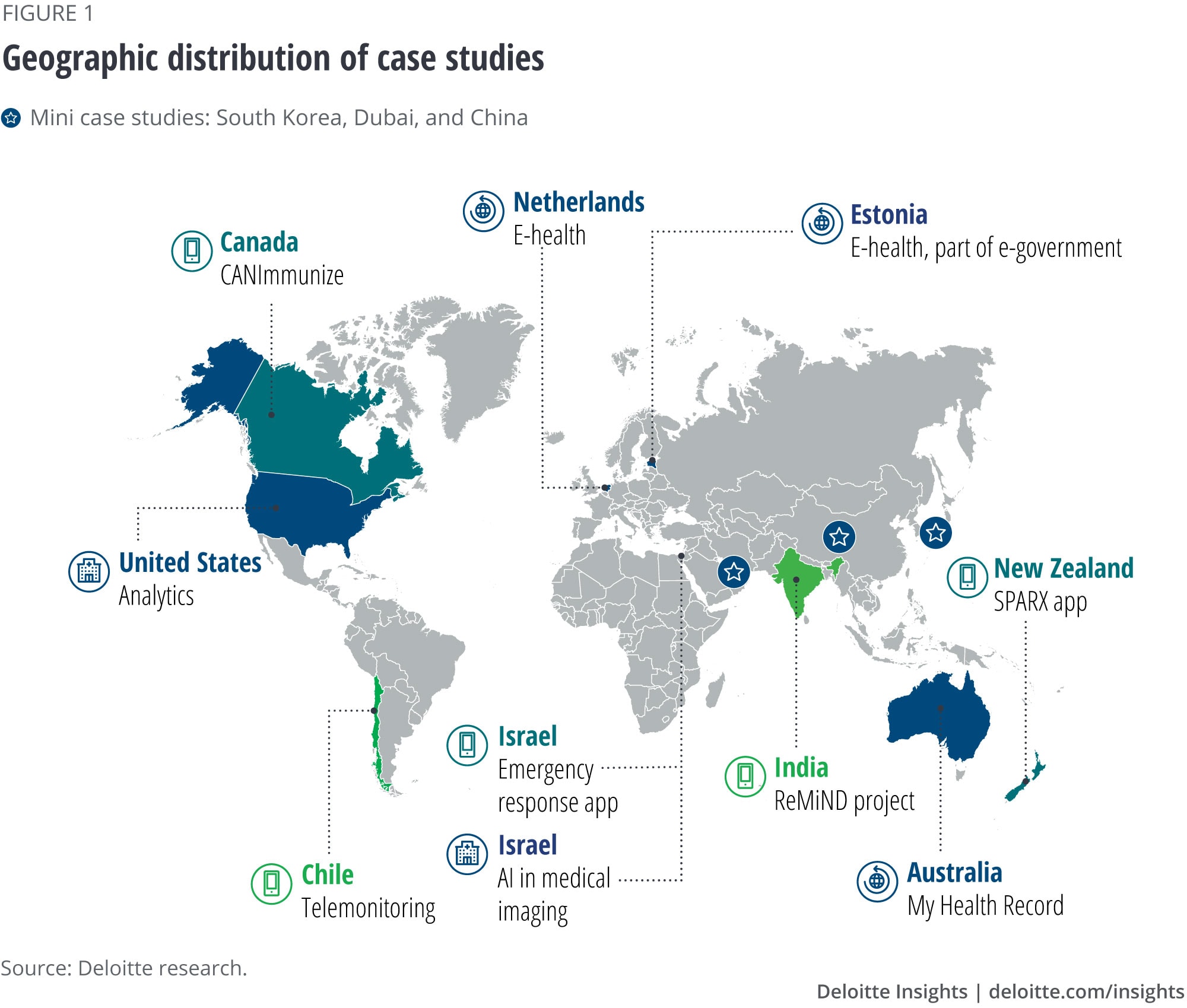
Health care of the future will be different from the health care of today
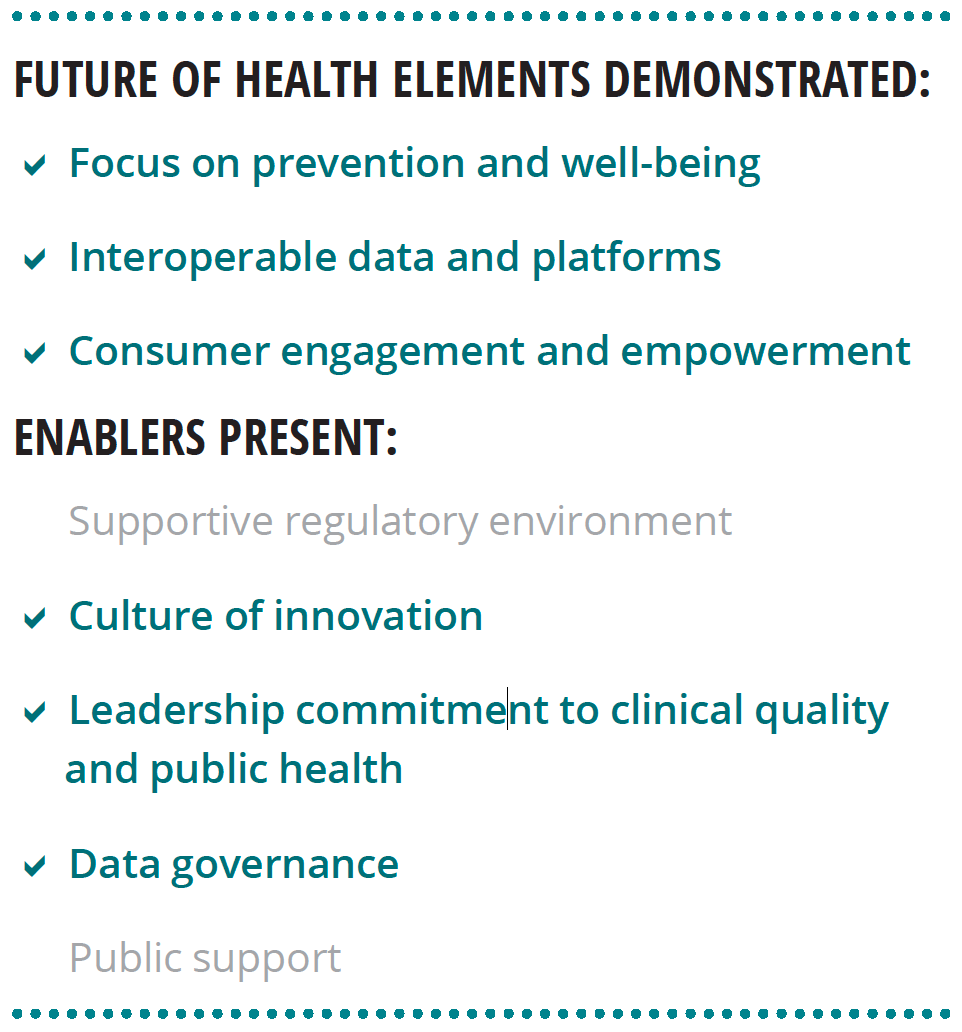
Deloitte envisions that health care of the future will be different from today’s in three distinct ways:
- Care will shift away from illness to prevention and well-being.
- Interoperable data and platforms will enable the free and secure flow of data on individuals, populations, institutions, and environment; insights from this data will inform real-time decisions about health.
- Increased consumer engagement and empowerment will drive attitudes and behaviors toward greater ownership of one’s health.3
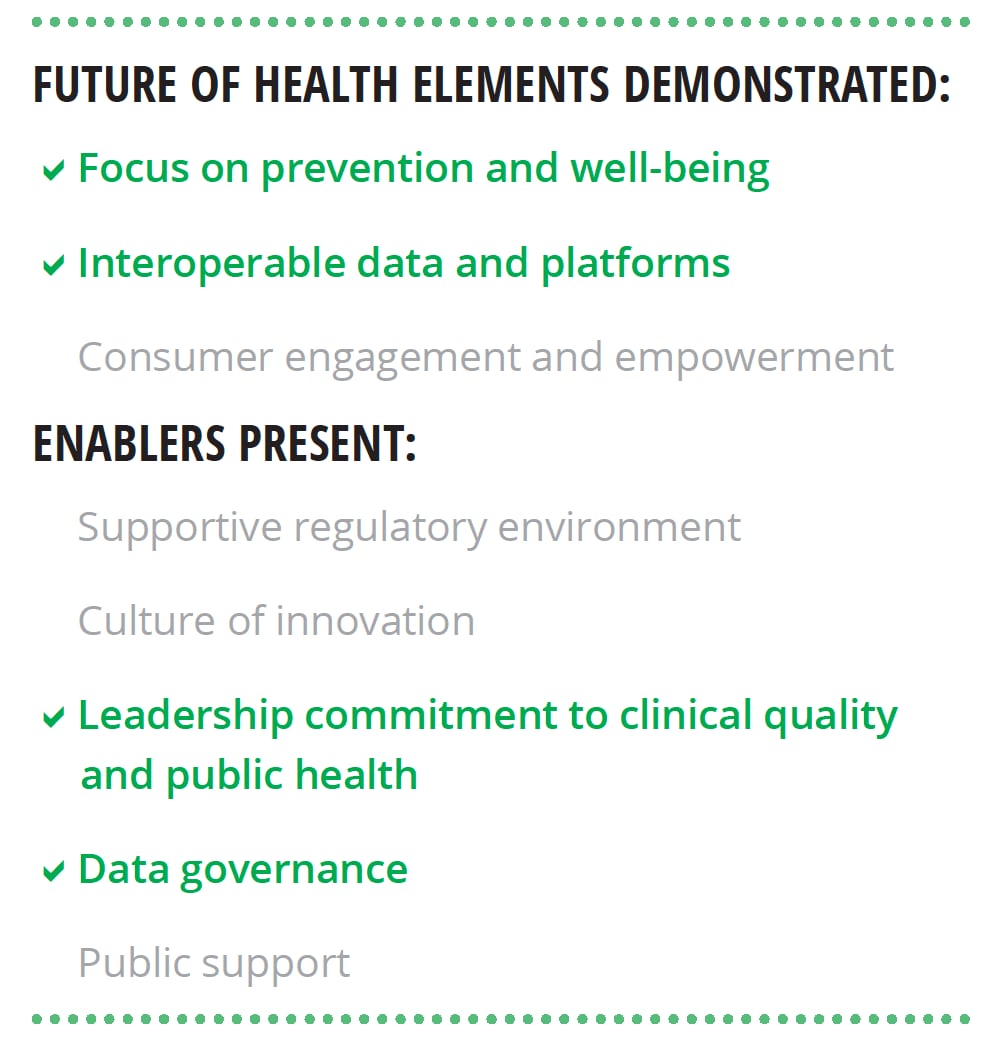
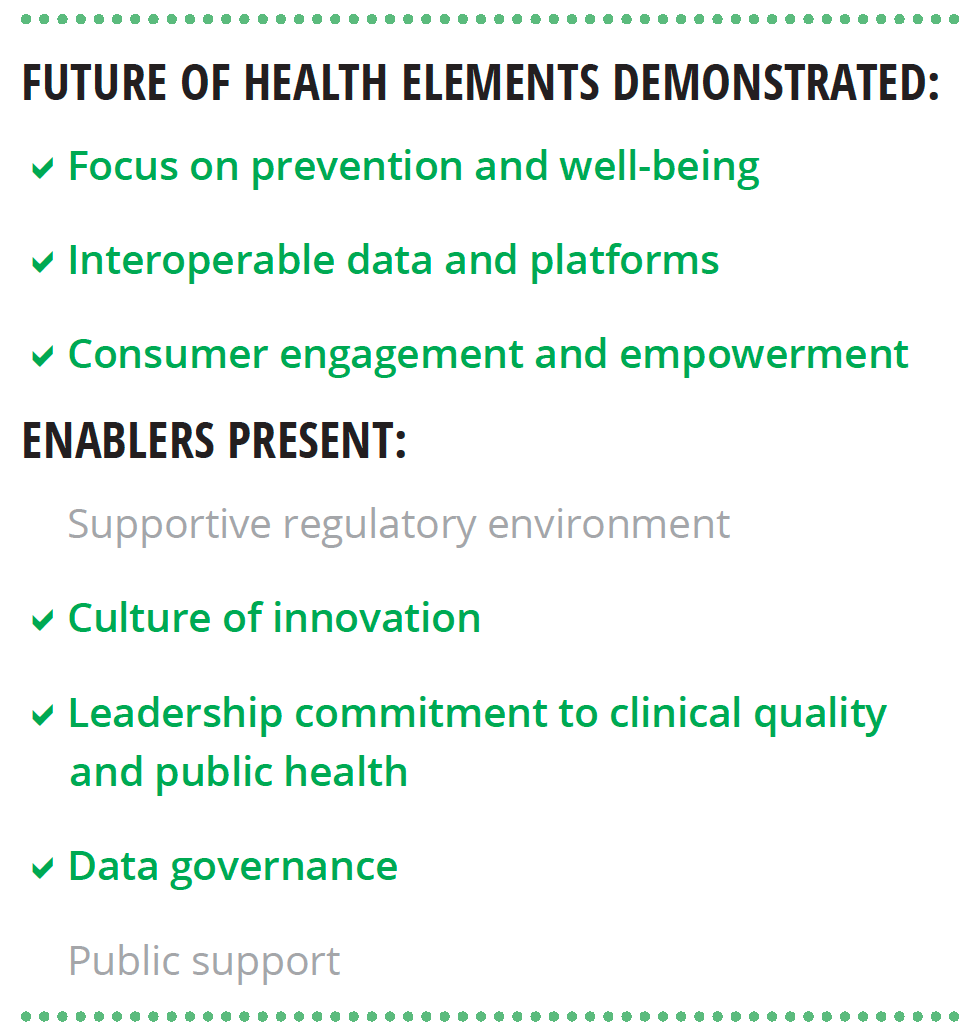
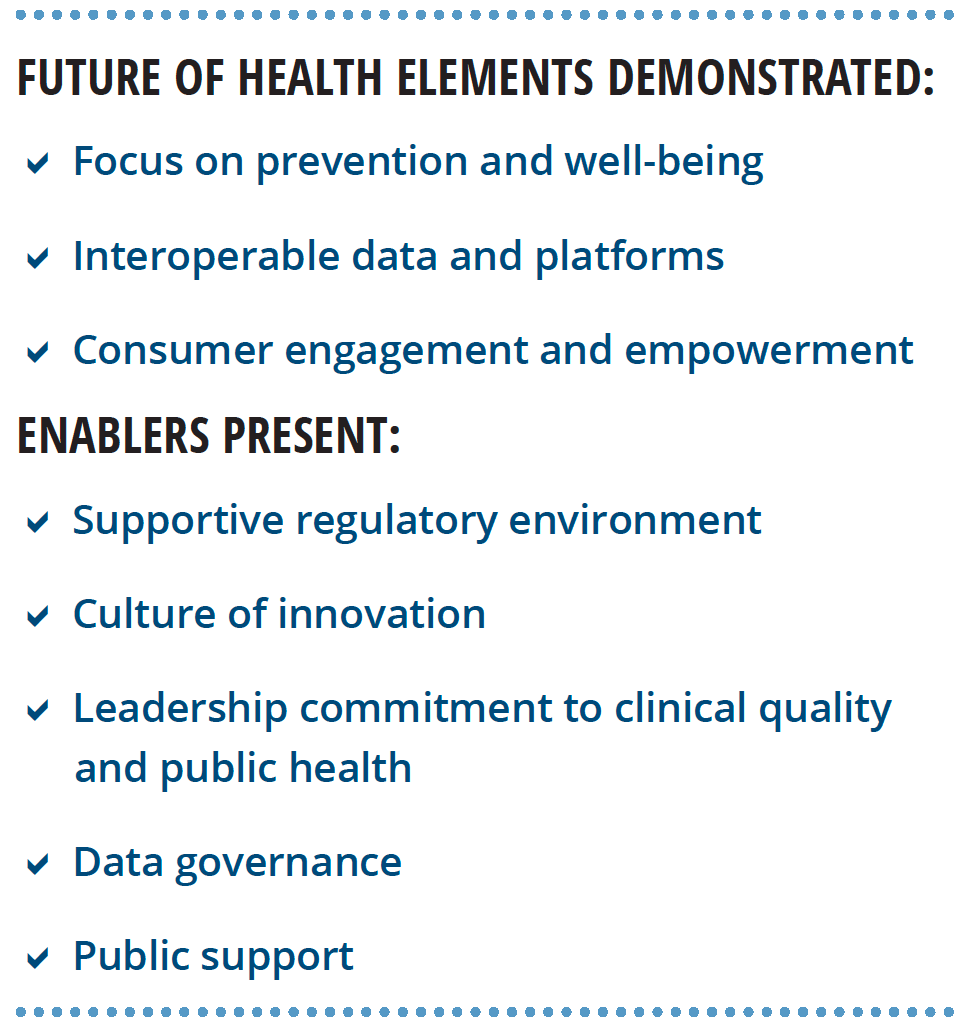
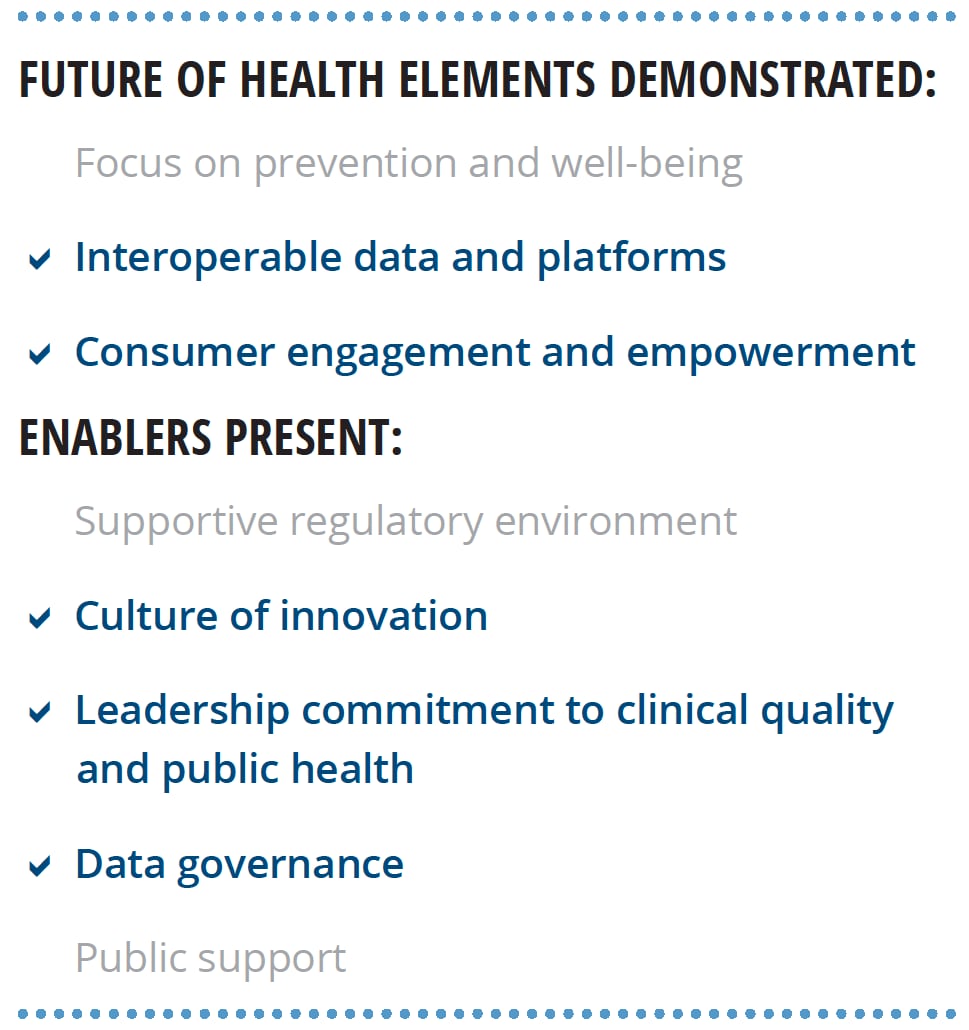
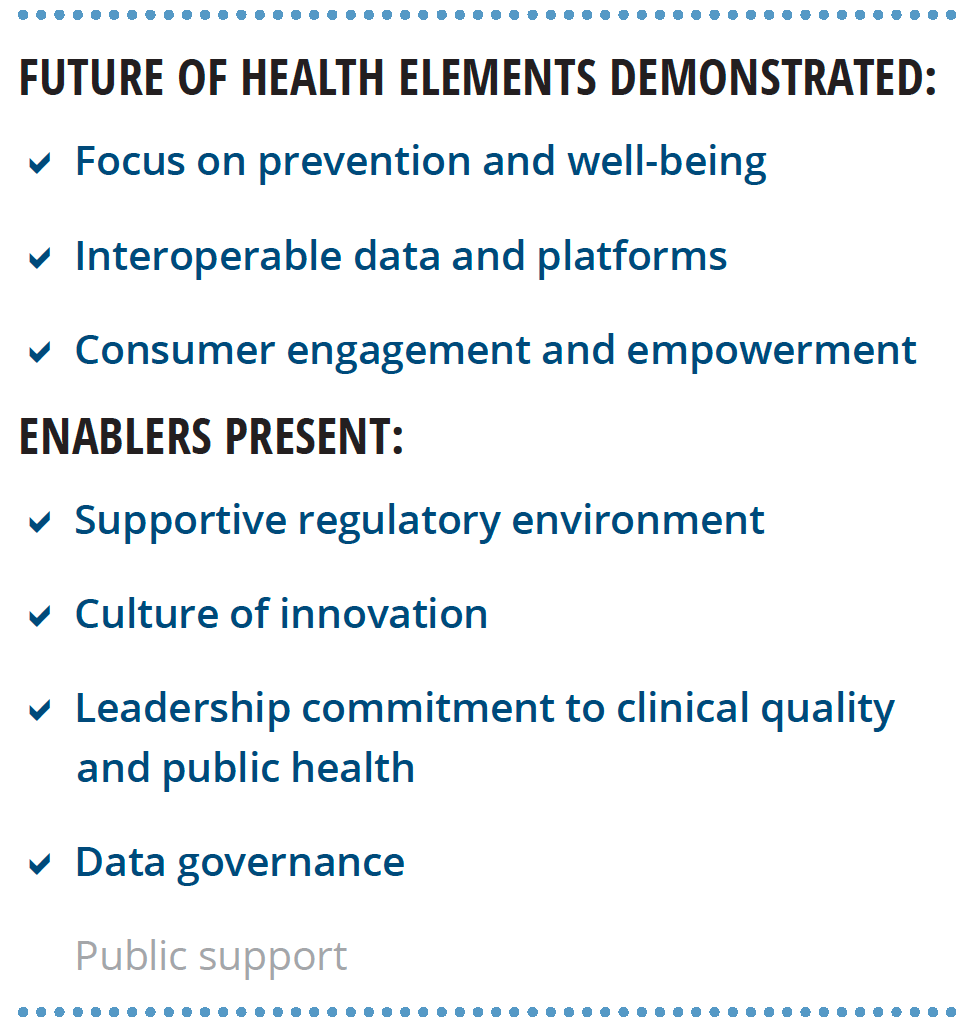
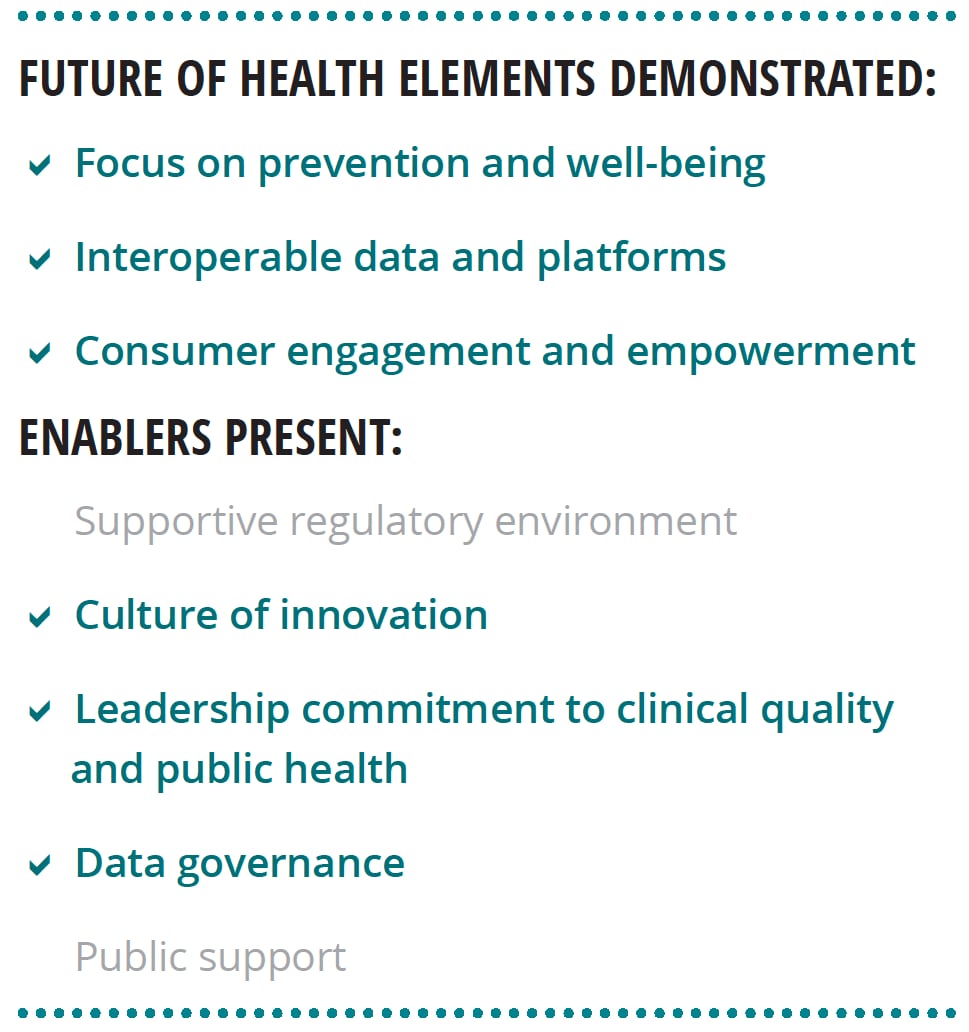
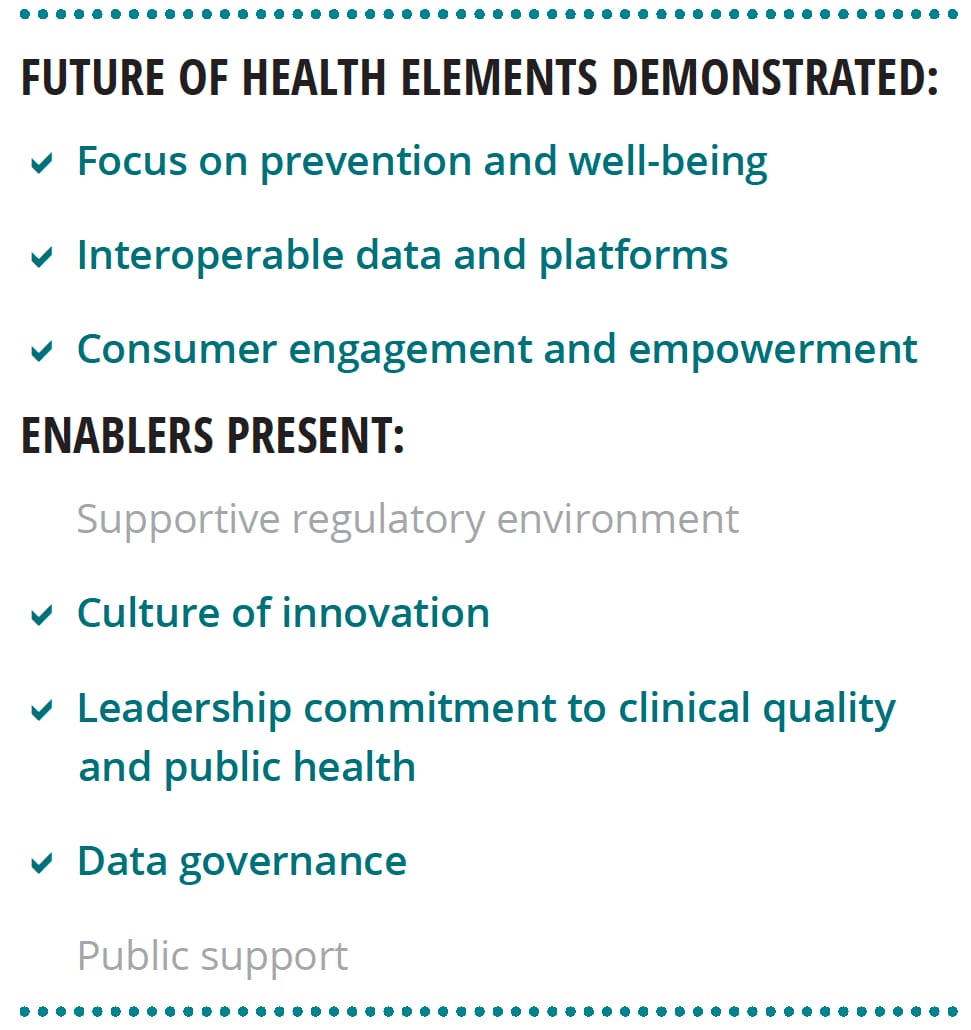
Our research lends support to Deloitte’s view of the future of health. We interviewed six world-renowned HIT experts about the characteristics of the future health care system, the technological capabilities needed to achieve that future, and nontechnological enablers that should be present. Most of the findings from these interviews align with Deloitte’s perspective (see figure 2).

Experts stressed that the transformation to the health care of the future requires more than just technology, it should include:
- A supportive regulatory environment
- A culture of innovation
- Leadership commitment to clinical quality and public health
- A foundational data governance framework
- Public support grounded in digital and health literacy and trust in institutions
Case studies
Each case study in our research offers elements of the future vision and illustrates how technological and nontechnological enablers contributed to success. We have organized the case studies in three sections, according to the future of health themes that they represent best:
- Focus on prevention and well-being
- Interoperable data and platforms
- Consumer engagement and empowerment
For each case study, we also highlight the nontechnological enablers that contributed to the success.
Focus on prevention and well-being
Initiatives in this section show how digital technologies help improve access to care and well-being for underserved populations.
The ReMiND project in rural India was designed to address deficiencies in an existing community health program for pregnant women and new mothers. To achieve the project goals, technology developers had to account for users’ low literacy skills and mobile phones without advanced features. However, the biggest contributor to success was change management: modifying meeting formats for program staff and using data to drive community health workers’ behavior change so they could accomplish better outcomes.
In the AccuHealth example in Chile, AI helps identify high-risk patients with chronic conditions most likely to benefit from a health-coaching intervention. Remote monitoring enables interventions almost in real time: Health coaches contact monitored patients within one to four hours of an abnormal biometric reading.
In both programs, digital technology delivers timely prompts and insights to the users, motivating behavioral change, which in turn contributes to saved lives, better health, and lower costs.
India: mHealth to improve maternal and newborn health
Technology and change management improve a community health worker program
The Reducing Maternal and Newborn Deaths (ReMiND) program in India demonstrates how a simple mHealth application can improve care delivery at the community level.
Of note
ReMiND’s impact on maternal and infant health is significant:4
- Since implementation, knowledge of pregnancy and delivery danger signs increased in women who were part of the ReMiND program.
- Studies also show a 12.7 percent increase in iron-folic acid consumption; a rise in identification and self‑reporting of complications during pregnancy (12.5 percent) and after delivery (15.5 percent); and more blood pressure, ultrasound, and abdominal checks.
- Overall, the program is projected to prevent 16,918 maternal (16.4 percent) and 119,646 infant (5.2 percent) deaths over a 10-year period across Uttar Pradesh.
In 2006, the Indian government launched the Accredited Social Health Activists (ASHAs) program to reduce maternal and infant mortality in rural communities. As part of the program, female community health workers (called ASHAs) visit pregnant women and new mothers to provide services such as nutritional counselling or referrals to hospitals for childbirth.
However, the ASHA program did not have the same impact in the state of Uttar Pradesh (which has historically high maternal and infant mortality rates)5 as in other parts of the country. To address the deficiencies of the program in Uttar Pradesh—limited training, inadequate job aid for ASHAs, weak supervision, and a lack of role clarity—Catholic Relief Services and its partners launched the ReMiND project.
ReMiND has two key components that strengthen community-level systems around maternal and newborn health:
Technology for data capture and reporting
- The mHealth app provides a user-friendly interface for ASHAs and their supervisors.
- For ASHAs, the app serves as a digital job aid to counsel women and families about prenatal care, delivery, and postnatal care. The app takes ASHAs through a checklist of health behaviors, prompting them to counsel young mothers and families on any steps not taken or considered.
- For the supervisors, the app serves as a tool to monitor ASHAs’ workloads and identify performance gaps so they can help ASHAs close those gaps.
Change management: Staff meetings and ASHA performance
- Under ReMiND, large ASHA meetings (involving more than 100 ASHAs, supervisors, and local government officials, leaving little time for problem-solving and sharing of experiences) were broken down into smaller events involving 20 ASHAs and a supervisor.
- Custom reports generated by ReMiND are used to talk to ASHAs about their performance and to solve problems.
In the first two years since the launch of ReMiND, 15 percent more pregnant women received a visit from an ASHA, and the percentage of low‑performing ASHAs decreased from 61 to 19 percent between 2011 and 2013.6 After the introduction of the app, women were 28 percent more likely to receive a counseling visit from ASHAs on twice as many topics.7
Catholic Relief Services attributes ReMiND’s success to its user-friendly app and structured interpersonal communications that helped ASHAs be more effective. While ReMiND was implemented at the community level, its benefits are potentially replicable at all levels of the health system (community, district, state, or even national).
Chile: AI and remote monitoring to improve chronic disease management
Technology helps health coaches target high-risk patients
Chronic diseases are responsible for an estimated 41 million deaths worldwide and the cost of chronic disease is enormous. In the United States, for example, caring for individuals with chronic conditions accounts for up to 66 percent of all national health care expenditures and 98 percent of total Medicare expenditures.8
In Chile, which has close to 5 million people with chronic conditions,9 AccuHealth (a health management company) uses AI-powered remote monitoring to help in the management of these patients. Unlike traditional disease management companies, AccuHealth performs real-time remote monitoring, as its AI stratifies patients to ensure that health coaches focus on high-risk patients and those in immediate need of intervention.
Of note
In Chile, AccuHealth uses two models to work with public and private payers:
- Fee for service: For a small fee per member per month, AccuHealth manages the entire population. This involves identifying and segmenting patients based on cost and disease complexity. AccuHealth can optimize the frequency and intensity of patient monitoring: for example, high-risk patients are monitored daily and more intensely, whereas low-risk patients are monitored at less frequent intervals.
- Shared savings:10 AccuHealth only enrolls high-cost patients. The actual cost per patient before enrolling in health coaching serves as the baseline. AccuHealth and the payer share savings based on the difference between actual expenditures and the cost baseline.
One of the biggest learnings has been the importance of human interaction:
- With AccuHealth kits and education, patients have all the tools to self-monitor and follow their treatment protocol. However, even with these tools, patients who self-monitored followed their protocol only 40 percent of the time. But once a health coach called and nudged them, adherence doubled to 80 percent, typically within one hour of an out-of-range reading.
- The degree of loneliness and social isolation in chronic patients, particularly the elderly, was another surprise. The company has added a full-time psychologist and nutritionist to provide holistic care.
AccuHealth’s kits consist of sensors and tablets that guide patients through biometric data collection (blood pressure, glucose levels, weight, and other indicators) and quick survey questions. The kits can be customized for different conditions (diabetes, hypertension, chronic obstructive pulmonary disease, and even post acute care) and use on-market clinically validated devices.
Trained on deidentified records of 2.4 million Chilean patients,11 AccuHealth’s algorithm segments patients based on health trends and psychological and sociological profiles to identify high-risk patients. This enables health coaches to concentrate on those for whom the impact of monitoring is likely greatest, decreasing the cost and effort involved in managing populations.
To assess clinical impact, AccuHealth conducted a study of 4,000 diabetics using its solution and measured A1c blood sugar and blood pressure levels at study start-up, monthly, and after a year. Results showed a 1.5-point reduction in A1c from the sixth month onward. AccuHealth estimates this translates into a 20–40 percent decrease in medical complications for chronic patients and a 30 percent decrease in costs over the next 10 years.12
AccuHealth reports its solution has led to a 32 percent reduction in inpatient and 15 percent reduction in emergency visits in a payer’s population. Additionally, it has led to a 41 percent decrease in costs associated with medical leaves, a major expenditure for payers in Chile. On average, private payers see a 35 percent savings from the AccuHealth solution.13
AccuHealth runs five full-scale and 10 pilot programs across five provinces and intends to expand its reach to 100,000 Chileans in the medium to long term, by working with more public and private payers.
Interoperable data and platforms
In this section, we talk about how interoperable data and platforms allow for exchange of health information and analytics that help increase the speed and effectiveness of health interventions. The case studies fall under two categories:
- Systemwide platforms that serve as regional or national infrastructures for health data exchange and storage
- Technological solutions to support clinical initiatives at hospital systems
Systemwide digital health platforms. In the future, we expect data to flow seamlessly across platforms, creating new ways for consumers and care providers to proactively collaborate while supporting a system of wellness. Systemwide platforms are huge undertakings, particularly in view of numerous legacy systems that impede interoperability, and certain basic capabilities must be in place to create a foundation for data-sharing.
For instance, achieving interoperability requires changes in clinical documentation practices at the organization and user level, as well as complex interfaces among clinical IT systems, even when standards are in place. Building its e-health system from the ground up, before providers had a chance to invest in their own electronic systems, Estonia may have benefited from early adoption. Australia and the Netherlands represent a more typical scenario where multiple legacy systems, EHR platforms, and organization-specific conventions have evolved as barriers to interoperability. To support interoperability, all three countries have implemented a system of unique IDs for patients, providers, and organizations, as well as strict authentication rules.
Typically, a new legal and regulatory framework is required to support digital health efforts. The pace and scale of adoption often hinges on a national decision of opt-in or opt-out for consumers’ participation in data sharing. An opt-out approach has been shown to increase adoption. Estonia has adopted opt-out from the beginning, without much controversy. Australia’s move from opt-in to opt-out was a solid course correction that should lead to higher usage, whereas the Netherlands may see a decline in usage after switching to an opt-in model.14 To gain public and political support for opt-out, legal and technological safeguards of data privacy and security are necessary. All three countries have incorporated these safeguards. Before connecting to a national network, providers must demonstrate that their IT systems meet technical and security requirements. Consumers can authorize and restrict access for certain providers, restrict access to portions of the record, and close the record altogether. The systems generate access logs, so consumers know who viewed or contributed to their record.
As is often the case with technology, an if you build it, they will come approach is unlikely to materialize. Achieving a critical mass of users is a common challenge. With providers, incentives and regulatory mandates are common approaches, whereas with consumers, opt-out is a good first step. Ongoing public awareness campaigns serve as reinforcement. But most importantly, technology should deliver value to users; this ensures not just nominal adoption, but actual use.
Estonia: Early adopter of e-health
E-health as part of a broader e-government strategy can deliver cost-effective solutions
Known worldwide for its e-government services in tax, voting, health care, education, and public safety,15 Estonia began to develop e-health infrastructure in the early 2000s and introduced the first e-health services for consumers in 2008.16
Initially, the e-health system had four major components:
- The electronic health record for providers to record patients’ diagnoses, lab tests, procedures, and prescriptions on a centralized database, which enables providers across the country to access patients’ health records. Today, EHRs are widely adopted by providers, and more than 95 percent of health data is digital.17
- Digital registration services to support digital referrals and patient appointment scheduling.
- The digital image program, an archiving and communication system for collecting and exchanging medical images.
- Digital prescription for providers and pharmacies to issue and manage patient prescriptions.
Availability of technical expertise, continued commitment from the government, and the public’s digital literacy and trust in the government enabled Estonia to realize its e-government vision. An entirely new legal framework was required to support e-health, including mandated use of EHRs by providers, compulsory citizen ID cards, and data security and access requirements.
Of note
As of 2015, the total cost of Estonia’s national e-health project was approximately US$3 million.18 This equates to about US$10 per patient record, considerably lower than in other developed countries.19
The security of e-health in Estonia relies on blockchain technology and authentication with ID cards, digital signatures, separation of personal data from medical data, encryption of data, and monitoring of actions, allowing users to know who accessed their health data.
Having built a centralized data architecture and secure environment for data exchange, Estonia was well-poised to introduce additional e-health initiatives:
- Statistics services that use anonymized population-level data for public health research and policy recommendations.
- An e-ambulance system that enables emergency teams to access a patient’s medical history and make informed treatment decisions.
- Integration of a decision-support system with the e-prescription database that provides drug interaction warnings to prescribing physicians. An estimated 15–17 percent of prescriptions are changed in response to these warnings.20
Going forward, connecting the centralized database with other systems presents new opportunities:
- Integration with the Estonian Genome Center will enable personalized treatment. By the end of 2019, 20 percent of Estonians are expected to get genetic screening.21
- Integration with environmental indicators has the potential to link environmental factors to public health outcomes.
Netherlands: Moving toward a digital future despite setbacks
Support from providers keeps the system running
Netherlands had an early start with e-health, but faced headwinds along the way. In 2002, the Ministry of Health, Welfare and Sport funded the National Information and Communication Technology Institute for Healthcare (NICTIZ) as a centralized body of expertise on e-health. NICTIZ was tasked with establishing a nationwide digital infrastructure (AORTA) for secure and reliable exchange of medical data among providers.
Completed in 2011, AORTA contained records of one in two Dutch patients.22 However, in response to political pressure, the system moved from patient opt-out to opt-in, the records collected up to that point had to be destroyed, and the responsibility for e-health implementation was transferred to the industry.
Of note
NICTIZ’s research suggests changes in culture and care processes are needed to accelerate adoption of e-health:23
- As of 2018, only 2 percent of all patients had online access to the patient records kept by their GP, and 8 percent for medical specialists. Among people with a chronic condition, these proportions were 4 and 7 percent.
- Only one in nine people with a chronic condition reported having used a device that regularly measures health values and shares them with a health care provider.
- Adoption of e-health applications is slow in the following circumstances:
- Use of applications requires actions from both patients and providers (such as remote monitoring).
- The added value is unclear: While half of all consumers would like access to their medical records, two in three physicians experience or expect negative effects from such access.
- There is no sense of urgency to use the application. Between 45 and 60 percent of health care providers are unaware of their organizations’ e-health vision or concrete objectives on usage and deployment.
Despite the setback, as of 2016, 92 percent of health care providers were connected to AORTA, some 11 million Dutch people (almost two-thirds of the population) opted to having their health data shared among providers, and around 150,000 messages were exchanged daily.24
Patient centricity moved to the top of the e-health agenda in 2014, with ambitious goals set for completion by 2019:25
- Forty percent of all people and 80 percent of the chronically ill should have electronic access to their own medical records.
- Seventy-five percent of chronically ill people and vulnerable elderly should be able to monitor things such as blood pressure and cholesterol, and share data with their provider.
- People requiring care at home should be able to communicate with their provider 24 hours a day via a screen.
Achieving these goals is taking longer than expected. Access to health information remains complicated for patients, as each health care organization operates its own patient portal.
The MedMij initiative championed by the ministry aims to accelerate the development of a central personal health record26 by fostering competition among technology developers. Several years from now, a patient should be able to choose one of several MedMij-certified apps to access their longitudinal data in one place and simplify tasks, such as making appointments and viewing test results.
Australia: My Health Record for a comprehensive view of one’s health
Integrating existing systems with new ones presents a promising solution
The national rollout of Australia’s My Health Record (MHR) was completed in early 2019, and initial evidence and anecdotal accounts suggest it is on track to deliver on its promise, to ensure that important health information is available when and where it is needed.27
During the disastrous floods in February 2019 in Townsville, MHR proved vital to patients cut off from their regular pharmacies and GPs.28 Patients were showing up at the pharmacies they could manage to get to, without prescriptions and without much knowledge of their medication. But the data in patients’ MHR gave pharmacists all the information necessary. It so happens that Townsville is in a pilot region, North Queensland, where MHR was launched in 2016, and most people in the area have an active and populated health record.29
MHR has considerable public health potential. As all clinicians involved in a person’s care begin to use and contribute data to MHR, it can help them coordinate care and order fewer duplicative tests and services. MHR could also serve as a personal health assistant reminding consumers about age- or condition-appropriate medications or tests (such as pap smears, vaccinations, or the A1c test). And it could even be a platform to document end-of-life wishes.30
MHR started in 2012 as the Personally Controlled Electronic Health Record. It was renamed My Health Record in 2016 and moved from an opt-in to opt-out consumer participation model to increase usage.
Of note
- A physician can directly upload prescribed medicines and a pharmacy can upload dispensed medicines to MHR, giving an overview of medication adherence, supporting medication reconciliation and safer use of medicines.
- Consumers can upload their own information,31 such as:
- Personal health summaries, including medications and allergies
- Emergency contacts
- An advance care directive custodian—a person or organization who holds their advance care directive
- Personal health notes not visible to health care providers, such as a health journal
- Child development notes, such as an achievement diary, personal observations, immunizations, and growth details
MHR does not replace the medical records created by clinical systems at physician practices or hospitals but rather contains a summary of relevant medical information. The Shared Health Summary generated by general practice contains a patient’s diagnoses, medications, allergies, vaccinations, as well as personal information such as age and sex.
Providers can upload to MHR other types of documents too:
- Event summaries
- Discharge summaries
- Medication records
- Medicines information view
- E-prescriptions
- E-referrals
- Pathology reports
- Diagnostic imaging reports
- Immunizations (as recorded in the Australian Immunization Register)
This data is automatically saved in the MHR by means of an interoperable data network that requires a high level of standardization of clinical documentation. Yet, fewer than 25 percent of health care providers use MHR guidelines for clinical terminology, and no national mandates exist to this effect.32
Clinical initiatives by hospital systems. Provider organizations find novel ways to use HIT to tackle inefficiencies and improve quality. Case studies in this section demonstrate how technology augments clinician decision-making and optimizes workflows.
Analytics coupled with clinician education has played a major role in transforming pain management at Geisinger in the United States. At the Sheba Medical Center in Israel, efficiencies created by AI support quality improvement by prioritizing critical cases in the radiologists’ workflow, reducing time to treatment and improving outcomes for patients with critical pathologies.
For both organizations, several important factors contributed to success: an integrated data and analytics infrastructure already in place; clearly defined goals a new solution would meet; culture that supports innovation and data-driven decision-making; and change management—such as user engagement, education, and feedback—to help achieve desired behavior change.
The United States: Changing pain management approach at a large health care organization
Geisinger Health System achieved a 66 percent reduction in opiod presecriptions since 2014

With 140 deaths per day from drug overdoses, the US Health and Human Services declared the opioid epidemic a public health emergency in 2017.33 In the years that led up to it, it had become increasingly apparent that prescription opioids played a big role.34 In 2012, the Geisinger health system, serving more than 1.5 million patients in Pennsylvania and New Jersey, decided to take a close look at the prescribing patterns of its own physicians.
Using its robust health information system, which captures longitudinal electronic health records and medical and prescription claims, Geisinger’s IT team built a controlled-substance monitoring dashboard that allowed population-level views and patient-level drilldown.35 Until they saw the data, high prescribers had no idea they were outliers.
Of note
It took one or two years of clinician education for things to start changing. A strong physician-led culture and a commitment to care quality ensured that all physicians embraced the feedback and reevaluated their prescribing behavior.
Today, Geisinger’s holistic pain management program includes physical therapy, mindfulness and meditation, exercise, acupuncture, diet and nutrition, and behavioral therapy.36
The cost benefit of Geisinger’s opioid prescribing program is hard to measure. In the short term, treating pain holistically may be more expensive than prescribing medications, but the risk of addiction is greatly reduced, and the outcomes and patient experience are improved. Geisinger may be in a better position than others to absorb the added cost, as most of its patients are customers for life and 40 percent are also insured through Geisinger.
With physician education aimed to encourage pain management approaches that do not rely heavily on opioids,37 opioid prescriptions fell from 60,000 per month in 2014 to 31,000 in 2017 and to under 22,000 in 2019.38 This would not have been possible without the organization’s dashboard obsession, powered by cradle-to-grave health data and the ability to turn that data into usable information.
Now, EHR integration with the state’s Prescription Drug Monitoring Program makes it possible to know if a patient has received or sought opioid prescriptions elsewhere. The EHR also limits the quantities and doses for new opioid prescriptions.39
With controls in place to minimize the risks for new addictions, the focus is shifting to treatment, which may prove the biggest challenge yet. Treatment is demanding, the risk of relapse is high, and many patients simply refuse treatment. Here, technology may once again serve as an enabler: A health information exchange that can ensure secure communications among the state’s treatment facilities is nearing completion.
Israel: AI in medical imaging
Reducing time to treatment by pushing critical cases to the top of radiologists' work list
Exponential growth of medical imaging in routine diagnostic practice, without a corresponding increase in the number of radiologists, has increased radiologists’ workloads: One US study estimates it increased from three images per minute per radiologist in 1999 to 16 in 2010.40

The growing demands on radiologists were acutely felt at Sheba Medical Center, Israel’s largest hospital campus. With 75 percent of patient care involving imaging and a conventional first-in-first-out workflow, radiology became the bottleneck, delaying diagnosis and treatment.41
The medical center looked to AI to improve efficiency. With Aidoc, a technology startup, the team focused on time-sensitive and potentially life-threatening conditions that can benefit from a quicker diagnosis.42 They started with brain imaging. When AI detects bleeding in the brain, that image pops up on a radiologist’s screen, pushing that case to the top of the work list so that the radiologist can review and confirm the diagnosis and return the results to the ordering physician, who can initiate immediate treatment.
Sheba’s radiologists welcomed the new tool and pushed for the development of AI solutions for other time-sensitive pathologies: pulmonary embolisms, lung nodules, fractures, and abdominal air. The software helps not only prioritize and reduce time to treatment, but also improves diagnostic accuracy, ensuring that no cases of life-threatening pathologies are missed.
Of note
Software developers spent weeks shadowing radiologists to understand their work, pain points, and communication flows. Several key insights informed the design of the tool:
- Radiologists serve internal clients, such as physicians in the ER and other departments, and imaging test results affect those physicians’ workflow.
- Solutions that require radiologists to exit their workflow and perform actions complicate user experience, particularly if such solutions are used infrequently.
- Although some false positives are expected, radiologists can lose trust in a decision-support tool that disrupts their workflow with false alarms.
Due to advanced IT and data security capabilities, Sheba was able to quickly roll out the solution to all radiologists, and its innovation-led culture contributed to physician engagement with Aidoc’s software developers and quick adoption of the technology.
With 96 percent accuracy, Aidoc’s solution has been shown to reduce turnaround time by 32 percent for critical cases.43 Studies are ongoing to evaluate the impact on overall length of stay, diagnostic accuracy, and turnaround time.
Going forward, Sheba and Aidoc are exploring the use of AI in:
- Nonemergent conditions such as oncology, to instantly detect, measure, and compare tumor size
- Additional modalities such as X-ray and MRI
- Opportunities to improve image quality, decrease MRI scanner time, reduce radiation dose, and standardize reporting
AI in diagnostic imaging shows promise around the world
Many countries are exploring AI’s potential in diagnostic imaging.
- South Korea: Gangnam Severance Hospital in Seoul tested Samsung’s S-Detect for Breast to determine whether AI in medical imaging could improve diagnostic accuracy. S-Detect analyzes ultrasound images for breast lesions and provides standardized reports and classifications. For doctors with experience of four years or less, the software increased the accuracy of diagnosis from 83 to 87 percent.44 In another study, the technology found that of the 192 breast masses, 37.5 percent were malignant and 62.5 percent were benign. When the cutoff was set at category 4a, the accuracy was significantly higher in S-Detect compared to the radiologist.45
- United Arab Emirates: In 2018, Dubai Health Authority (DHA) announced plans to use AI for chest X-rays, required for resident visas. The goal is to improve workflow, ensure faster image analysis, and automate reports. DHA has 19 medical fitness centers across the emirate for issuing and renewing visas. DHA will implement this technology across a few medical fitness centers and assess the feasibility of expanding this technology to others.46
- China: In an effort to address the shortage of doctors and to make their workload more manageable, Peredoc has built screening and diagnosis systems to aid detection and diagnosis of pathologies in the lung, liver, and breast. Using data from more than 180 hospitals, Peredoc software can read X-rays and CT scans, identify nodules, particularly in the lung, and highlight potential malignancies, at a much faster rate. Today, the system is installed in 20 hospitals in China. Reported accuracy:47
- Detection rate for two- to five-millimeter pulmonary nodules is 94.9 percent.
- Detection rate for five millimeter pulmonary nodules it is 99.2 percent.
Consumer engagement and empowerment
Initiatives in this section are early experiments that target large segments of the population and demonstrate the potential of mobile technologies to meet population health goals, by actively engaging and empowering consumers.
In Canada, parents responsible for tracking their children’s immunizations have a new tool to help with the manual process. The CANImmunize app is designed to increase immunization rates and accuracy of immunization records in a fragmented system by giving individuals control of their records and equipping them with evidence-based information. It also created a foundation for data exchange and vaccine surveillance at a national level.
In emergencies, rapid information can be the difference between life and death. The MyMDA case study from Israel demonstrates that making individuals a part of the care team can increase the quality and efficiency of the emergency response. By making it easier for consumers to share their medical history and nature of the emergency, the app helps make the emergency response team faster and better prepared.
The SPARX mobile game from New Zealand reveals how youth engagement can drive improvement in mental health. By gamifying the intervention designed specifically for teenagers with depression, the e-therapy mobile app has been successful at improving emotional resilience and increasing requests for face-to-face counseling, particularly among underserved indigenous youth. Health questionnaires included in the game generate evidence of effectiveness as the app is used.
Canada: Mobile app to improve vaccine documentation and adherence to vaccine schedules
Technology benefits consumers and public health researchers
In Canada, parents are often the custodians of their kids’ immunization records. But the system doesn’t make it easy. For one, it is possible to get vaccines at multiple types of providers (pharmacists, doctors, emergency departments) who might not share information with each other. Furthermore, immunization schedules differ across provinces: For instance, Quebec recommends the second dose of the measles, mumps, and rubella vaccine at 18 months, while Ontario does so at four to six years.48 And the system is problematic for national public health reporting too, due to different data standards in provincial immunization registries.
CANImmunize, a mobile app and web platform to help families manage immunization records, was born out of a chance conversation in 2011 between a mom and a public health researcher. After its initial launch in Ontario, the app was rolled out nationally in 2014, with support from the Public Health Agency of Canada. Today, the app counts 55,000 users of the cloud-based platform and 300,000 mobile downloads.49Available in French and English, the app enables users to:
- Receive personalized immunization recommendations according to 13 provincial and territorial schedules and load their immunization records in the app. Users can receive reminders for vaccines due and for vaccination appointments scheduled.
- Access evidence-based vaccine information in an easy-to-understand language.
- Educate children about vaccination using videos and games.
- Identify local outbreaks of vaccine-preventable diseases.
- Access immunization-related pain management tips.
Efforts are underway to scale up the app by enabling:
- Integration with providers’ EHRs
- Exchange of data between administering providers, vaccine registries, and the app, with the goal of real-time access and synchronized vaccine records at different sources
- Provider digital signatures authenticating vaccine administration
A pilot program in Ontario to access the provincial immunization registry through the app has shown promise and is a step toward moving immunization records digitally between provinces.50 Digital scanning of vaccine product barcodes, useful for product recalls and adverse event reporting, has been shown to be technically feasible.51
Of note
The Canadian Vaccine Catalogue (CVC) is the byproduct of the data science that went into developing the CANImmunize app. The CVC is a standardized vaccine terminology and regularly updated database of all vaccine products in Canada that can translate vaccine information for EHRs and information systems used by different provinces. The CVC helps achieve Canada’s vision of a national network of immunization registries and improve vaccine surveillance.
Collaboration with school districts helps increase public awareness about the app: When schools send out suspension letters for failing to provide up-to-date immunization records, usage spikes.52 Ottawa, Toronto, and Kingston schools accept immunization record submission through the app and other counties plan to do so soon.
In the future, CANImmunize has the potential to improve vaccination rates and surveillance among adults, and serve as a digital immunization passport. Digital technology can also address the vaccination needs of niche groups, such as bone marrow transplant recipients and cancer patients.
Israel: Transforming emergency medical care through mobile technology
Sharing economy principles applied to emergency response
Timely emergency medical response is often hampered by a lack of critical information, such as the precise location of the emergency, the patient’s medical history, and a clear understanding of the nature of the emergency. Emergency medical teams (EMT) across the world spend considerable time—a crucial resource in emergency situations—to gather this information, which leads to a delay in emergency response.53
Magen David Adom (MDA), Israel’s national emergency service, built a mobile app known as MyMDA to increase its efficiency in emergency responses. The consumer app is available in multiple languages and enables users to:54
- Quickly summon the EMT team through the mobile app.
- Automatically report geolocation to the EMT dispatcher via their smartphone’s geolocation feature.
- Give paramedics access to medical information. Users can enter vital medical information (e.g., pre-existing medical conditions and current medicines) by answering a health history questionnaire when setting up the app. Access to this information helps EMT and treating providers to make informed decisions. Currently the app is not connected to the national health IT network and relies on users to input their health data.
- Communicate the nature of a medical emergency. A live video feed or photos from the emergency scene can be a more efficient way to communicate information than a verbal description. This enables the dispatch center to assess the medical situation and provide lifesaving instruction to users. Moreover, MDA can dispatch an emergency response vehicle most suitable for the situation (e.g., a basic life support ambulance, mobile intensive care unit, armored ambulance, or a medevac helicopter).
- Track the response team. Users can track the ambulance route and estimate its arrival time.
Of note
The MyMDA app is part of a suite of mobile apps that not only creates connections and data exchange between EMTs, first responders, ICU teams at hospitals, and consumers in emergencies, but also enables peer-to-peer sharing of resources:55
With the data from the consumer and the first responder apps, the MDA technology can automatically call the five nearest first responders to the emergency scene. Trained as paramedics and first-aids,56 first responders use their own transport and typically take less than five minutes to arrive, which is several minutes before the ambulance.57
MDA can identify other MyMDA users carrying emergency medicines (such as EpiPens or insulin). If needed, the app can locate the closest user in possession of these medicines and ask them to bring the medicine to the site of the emergency. Upon arrival, the EMT team replaces their medicine.
Since its launch in 2016, the app has been downloaded more than 150,000 times and the emergency response time has been cut by 35 seconds.58
Early adopters were people with medical conditions anticipating the need for emergency care. Adoption by healthy people has been slower.
The next version of the app will give users the option to take advantage of all the extended features or just the basic ones. This way, people unwilling to provide their personal health information and national ID number can still use the app for calling, location sharing, and sending video feeds.
MDA executives have a vision for a new approach to emergencies:
- Country-specific emergency apps such as MyMDA will be preloaded on all new smartphones sold in corresponding geographies.
- MyMDA will be integrated with the national health IT system, and users’ health data will be accessible with their consent.
- All health workers will be registered as first responders.
New Zealand: Gamification to improve teen mental health
Behavioral e-therapy with a track record of effectiveness
With one of the highest teenage suicide rates globally59 and a shortage of counseling services, New Zealand harnessed an innovative e-therapy solution to reduce the impact of teenage depression.
Funded by the Prime Minister’s National Youth Mental Health Initiative, the SPARX app uses cognitive behavioral therapy to assist teenagers with mild to moderate depression and anxiety. Teenagers sign up and play as avatars exploring a 3D world to complete quests, meet new characters, play mini-games, and solve puzzles. Virtual guides instruct players on how to apply these new learnings to feel better and solve problems in real life.
Initially, mental health professionals were skeptical about SPARX’s effectiveness. But several studies have shown SPARX to be as effective as face-to-face counselling.60 For instance, in a 2012 comparative study (n=187) measuring the effectiveness of SPARX versus traditional counselling, SPARX helped more kids aged 12–19 recover from depression. Additionally, 43.7 percent achieved remission, compared to the 26.4 percent receiving traditional counselling.61
Of note
The app tracks user progress by administering a patient health questionnaire, PHQ-9 (a validated mental health assessment tool), as the player progresses through game levels. The ongoing assessment measures app effectiveness at the population and individual level by ensuring the app does not cause harm or create an opportunity cost (e.g., by keeping patients from seeking further help while they participate in an intervention that isn’t working). If scores remain low, the app prompts the player to use SPARX’s telephonic or text messaging service or seek face-to-face counselling.
The SPARX marketing efforts target counsellors, school nurses, and physicians, as well as parents and teenagers using a multichannel approach: events (e.g., annual polyfest) and traditional and social media. Due to the generational nature of the SPARX user base, the marketing approach needs to keep up with teenagers’ evolving media preferences.
As of May 2019, the app had more than 20,000 registered users in New Zealand. The SPARX initiative has resulted in multiple successes.
- Due to long wait times for face-to-face counselling, SPARX is used to build emotional resilience before teenagers see a counsellor.
- Indigenous Maori adolescents have higher suicide rates than non-Maoris. SPARX provides customizable avatars that Maori youths can relate to. The use of SPARX in this population was associated with an increase in requests for face-to-face counselling.62
- SPARX is licensed outside New Zealand—in Australia, Japan,63 and Canada.64
The SPARX team also described some of the challenges:
- Despite gamification, most users fail to complete all levels of the game. Only 4 percent have completed all seven levels.65
- Usage has been low among Pacific Island youths, another indigenous population impacted by poor mental health.
The team is in the process of building SPARX 2.0. New features could include direct connection for face-to-face services, data exchange with national databases (health care services and social security and education databases), APIs that will connect other apps onto the SPARX platform, and capabilities to conduct randomized clinical trials through the app.
Conclusions
Health information and digital technologies are helping to create the necessary foundation for the future of health. Technology can help organizations improve their existing business and reinvent themselves once they decide what roles they want to play in the future. Together with other organizational capabilities, technology can help improve behaviors, augment our thinking, and optimize processes, leading to better health and lower costs.
Some of the lessons learned from our case studies are:
- An overall vision, strategy, and specific goals should be clearly defined for a technological solution to be successful. As technology becomes more powerful and pervasive, HIT departments inside leading health care organizations are evolving from a supporting to a strategic function, enabling the foundational changes that can support future models of care.
- People are often the overlooked part of the people, process, and technology model. Change management should always accompany and even precede a technology implementation, to understand the impact on stakeholders, anticipate resistance, create buy-in, train users, and use their feedback for course corrections.
- Typically, a foundational HIT infrastructure must be in place before organizations can move forward with advanced initiatives. Such foundational capabilities include data exchange and integration, traditional analytics, data visualization, and security protocols.
We hope this report inspires readers to continue to think about what the future of digitally-enabled health care may look like, how organizations should prepare for it, and the role they will play in this future.
Appendix: Study methodology
We have used a multimethod approach to address the goals of this research, in three workstreams.
Workstream 1: Interviews with HIT experts
- We conducted interviews with six world-renowned experts in HIT and population health (such as executives at the World Health Organization, health care advisers to national governments, chief information officers or chief digital officers at large organizations with a track record of innovation) to build out the elements of the future health care system, technological and nontechnological requirements to achieve this future, and to get their suggestions for promising examples of organizations implementing technological innovations.
Workstream 2: Environmental scan to identify promising case examples
- We scanned the available literature to identify candidate organizations for case studies. Our first set contained 38 examples. Initial criteria were:
- Novel, interesting use of technology, future orientation
- Variability of types of technology and data sources, diseases/conditions, inpatient and outpatient focus, types of stakeholders involved (private, public, academic, etc.)
- Presence of evidence of effectiveness or outcome measures
- Wide geographic representation
- We narrowed down case examples for further research by applying selection criteria and conducting discussions with internal subject matter experts. After applying the criteria below, our set had 15 examples.
- Availability of evidence, proof of effectiveness
- Scale:
- Size of the health system, size of the population affected
- Public health impact, either current or potential
- The initiative is part of a comprehensive population health strategy as opposed to a one-off effort; there is commitment to sustain and scale the initiative
Workstream 3: Further research on selected case examples
- We conducted semistructured interviews with experts from organizations that are the subject of the case study (e.g., health systems or hospitals, government agencies, technology developers, academics evaluating technology impact).
- In parallel, we performed additional secondary research to supplement findings from the environmental scan.
- We then reapplied selection criteria to final case studies.
- We arrived at a prefinal set of 12 that was further narrowed down to 10.
- We supplemented this final set with three mini-examples (see sidebar, “AI in diagnostic imaging shows promise around the world”) identified during the environmental scan.
© 2021. See Terms of Use for more information.
Learn more about data and technology in health care
-
Health care Collection
-
Virtual health care Video
-
Predictive analytics in health care Article5 years ago
-
Creating a treasure trove of data for health plans Article5 years ago
-
Health systems have a growing strategic focus on analytics Article6 years ago
-
Showing cybersecurity's value to health care leadership Article5 years ago












