The future of biopharma Reimagining traditional business models in 2040
23 minute read
23 March 2020
Shifts in how diseases are diagnosed and prevented, custom treatments, curative therapies, digital therapeutics, and precision intervention could upend current business models in biopharma.
Executive summary
Over the next 20 years, we expect biopharma business models to be reshaped by five forces—from inside and outside of the industry—that will likely require current players to evaluate shifting markets and determine how they will compete. Biopharma companies will continue to develop new ways to treat and cure a wide range of diseases. However, actionable health insights, driven by radically interoperable data and artificial intelligence (AI), can help clinicians and consumers identify illness much earlier than we do today. Vaccines and other early interventions could prevent a greater number of diseases from developing in the first place. Other illnesses might be prevented through nonpharmacological treatments. Shifts in how diseases are identified, prevented, treated, or cured may lead to fundamentally different business models for traditional biopharma companies and new entrants.
Learn more
Explore the health care collection
Learn about Deloitte’s services
Go straight to smart. Get the Deloitte Insights app.
Researchers from the Deloitte Center for Health Solutions interviewed 14 thought leaders (futurists, venture capitalists, digital health leaders, and academics) to find out how they expected the identification, prevention, and treatment of disease to evolve between now and 2040. Through these interviews, five forces emerged that could alter the course of the biopharmaceutical sector. These forces represent both opportunities and threats to incumbents. They include prevention and early detection, custom treatments and personalized medicine, curative therapies, digital therapeutics, and precision intervention. Interviewees noted that disruption is already taking place in the industry, and they tried to project where those threats might lead over the next 20 years.
The forces of change highlighted in this paper are likely to reshape, if not shrink, the market for biopharma products. Biopharma companies should consider the potential for disruption by these forces as they redefine the types of products and solutions they will offer, where they will compete, and the new capabilities they will require. Company leaders should develop strategies to counter potential threats and take advantage of the short- and long-term opportunities that emerge in this changing environment. Organizations that ignore these forces and maintain the status quo could wind up shrinking in parallel to the demand for drug interventions to manage symptomatic diseases.
Introduction
The future of health that we envision in 2040 will be a world apart from what we have now. Based on emerging technology, we can be reasonably certain that digital transformation—enabled by radically interoperable data, AI, and open, secure platforms—will drive much of this change. Unlike today, we believe care will be organized around the consumer, rather than around the institutions that drive our existing health care system.
By 2040 (and perhaps beginning much before), streams of health data—together with data from a variety of other relevant sources—will likely merge to create a multifaceted and highly personalized picture of every consumer’s well-being. Many digital health companies are already beginning to incorporate always-on biosensors and software into devices that can generate, gather, and share data. Advanced cognitive technologies could be developed to analyze a significantly large set of parameters and create personalized insights into a consumer’s health. The availability of data and personalized AI can enable precision well-being and real-time microinterventions that allow us to get ahead of sickness and far ahead of catastrophic disease. By 2040, health is likely to revolve around preventing some diseases from happening, and curing others. Many fewer people would have long-term conditions with continued need for medication to treat symptoms.1
In this future of health, the era of blockbuster drugs that treat large populations will likely wane. Instead, the biopharma sector is on the fringe of an era where hypertailored therapies are developed to cure or prevent disease rather than treat symptoms. Twenty years from now, rather than picking up a prescription at the pharmacy, personalized therapies based on a diverse set of a patient’s characteristics including their genomics, metabolome, microbiome, and other clinical information might be manufactured or compounded just in time through additive manufacturing.
How might exponential advancements in science and technology impact biopharma companies? What are some examples of disruption already happening in the market, and how quickly is change likely to occur? To find out, researchers from the Deloitte Center for Health Solutions conducted interviews with 14 forward-thinking industry experts (for more details see the sidebar, “Methodology”).
Methodology
The Deloitte Center for Health Solutions conducted telephone interviews with 14 industry experts including researchers, academics, futurists, investors, and former biopharmaceutical company executives from September to December of 2019. In addition, we conducted secondary research to identify startups and established companies that are already beginning to disrupt the biopharma sector.
A note to readers: This research was conducted before the novel coronavirus (COVID-19) became a focus for drug and vaccine development. At the time of publication, academic and biopharma researchers were increasing data transparency, leveraging accelerated regulatory pathways, and setting up adaptive trials to develop and test drugs and vaccines to address the pandemic. It is too early to opine on the timing and impact of those activities and how the forces we describe in this paper might accelerate or change how biopharma responds to pandemics in the future. We look forward to reporting about innovative approaches and lessons learned from this situation.
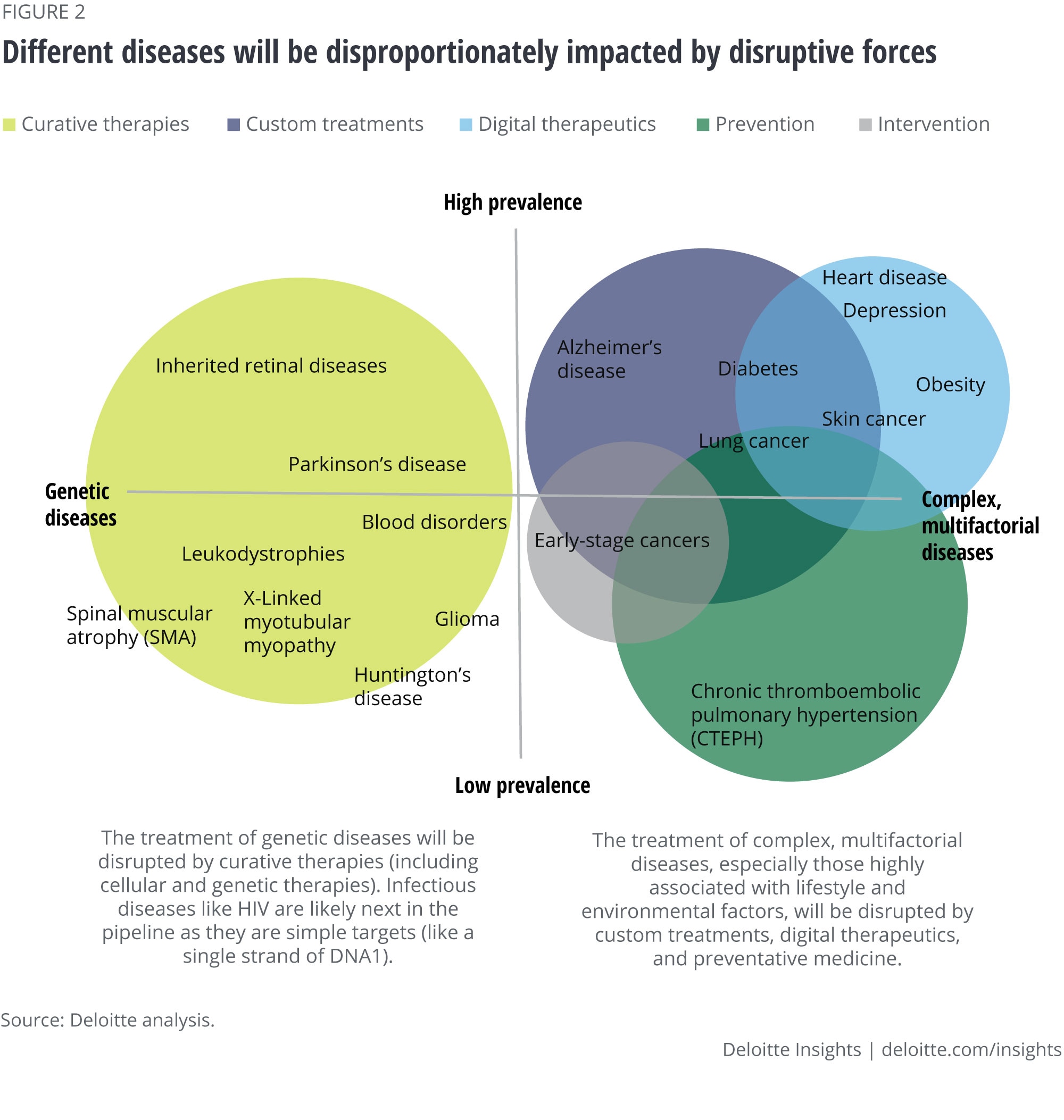
The five forces
Our interviews confirmed that five forces are beginning to impact biopharma companies but are likely to disrupt the sector more dramatically in the years ahead. Most of our interviewees agreed with our vision for the future of health that these forces—driven by players inside and outside of the biopharma sector—could have a seismic impact on biopharmaceutical companies and the patients they serve. The five forces, listed in order of potential disruption to the traditional scope of the biopharma industry, are:
Prevention and early detection: Vaccines and improvements in wellness could help prevent disease, making treatment for some diseases no longer necessary. Advances in early detection will likely enable interventions that halt diseases in the earliest stages—before they progress to more serious conditions.
Customized treatments: Personalization in medicine—driven by data-powered insights—could effectively match patients with customized drug cocktails, or design therapies that would work for just a few people, or even a particular person (i.e., “n of 1”).
Curative therapies: As with prevention, treatments that cure disease could reduce or eliminate the demand for some prescription medicines. Developing, marketing, and pricing these curative treatments could require the biopharma sector to adopt new capabilities.
Digital therapeutics: Increasingly effective and scalable nonpharmaceutical (digital) interventions—including those focused on behavior modification—might also reduce or eliminate demand for medications.
Precision intervention: Increasingly sophisticated medical technology—such as precise medical intervention enabled by robotics, nanotechnology, or tissue engineering—could reduce the need for pharmaceutical intervention.

As we move toward the future of health, biopharma incumbents should consider new strategic investments to position themselves for success. The changes that we see on the horizon will likely require biopharma companies to consider new types of markets, alternative business models, or a complete change in how they define what work they do.
Force no. 1: Prediction and early detection
Prevention of disease and a shift to wellness is a core pillar of Deloitte’s perspective on the future of health. We expect that over the next 20 years, we will be able to detect some diseases—and prevent them from advancing—possibly even before symptoms surface. Today, for example, clinicians can detect the early stages of melanoma much earlier than in the past, and early treatment can eliminate the disease completely. Not intervening until it metastasizes results in complications and expensive treatments. Early detection of other types of cancer could reduce or eliminate the need for future therapies.
Our interviewees suggested that other chronic diseases, such as Alzheimer’s disease, diabetes, and rheumatoid arthritis, might be treated more effectively if they are identified early. They also noted that earlier detection of a disease—or an understanding that a disease might be avoidable—could motivate people to adopt healthier lifestyles.
Vaccines: Vaccines are being developed that include but expand beyond childhood or common infectious diseases. In 2006, the US Food and Drug Administration (FDA) approved the first vaccine to prevent the human papillomavirus (HPV), which can lead to cervical cancer.2 If used widely enough, the vaccine could substantially reduce and potentially eliminate cervical cancer. Two other examples of virally transfused cancers include the human T-lymphotropic virus type 3 (HTLV-3) and the hepatitis B and C viruses, which can cause liver cancer. Over the next 20 years, more vaccines could be developed that prevent more types of cancer. The industry is beginning to think differently about the impact vaccines could have on other types of cancer by using the patient’s own immunity against self-antigens that are expressed in tumor cells. While these too are vaccines, they bear no resemblance to vaccines that prevent an infectious agent.
In the years ahead, the delivery of vaccines could move away from the traditional syringe as a delivery device. In 2017, the National Institutes of Health (NIH) funded a study that showed how an influenza vaccine could produce an effective immune response through a bandage strip lined with a patch of painless microneedles that are just long enough to penetrate the skin. The needles, which are painless, dissolve minutes after the vaccine is delivered and the bandage can be thrown away.3 In the future, such patches could be mailed directly to patients who could administer a vaccine themselves, increasing access and adoption.
Genetic testing: Whole-genome sequencing is a powerful and revolutionary tool. In fact, the genetic testing market is expected to reach US$17.6 million by 2025 with a CAGR of 11.3 percent.4 Early detection of a disease, or the ability to determine who might be genetically predisposed to a disease, could make it possible to cure an illness in the early stages or prevent it altogether. As gene sequencing, our ability to interpret the data, and gene-editing tools become more sophisticated, we expect to see an increase in early detection and preventative or curative interventions.
Some of our interviewees noted that the ability to use genetic information to identify patients at risk for developing disease could create an opportunity for biopharma companies.
“It is conceivable that if biopharma companies, NIH, academia, and others don’t ramp up their ability to cure disease, we could have a population of people who have an early diagnosis but no cure.”
—Academic researcher
There was also some discussion that our ever-growing knowledge of genetic risks could lead to under- or over-treatment. Case in point: In December 2019, the Wall Street Journal reported that seven women from the same family underwent major surgeries after a genetic test determined they all had an elevated risk of developing breast cancer.5 Since then, we’ve learned the increased risk associated with this particular gene is not quite as high as previously thought. The surgeries, which led to complications, might not have been necessary.
Early detection: Emerging technologies could dramatically improve our ability to detect disease in its earliest stages. For example, the home bathroom of the future might include a smart toilet that uses always-on sensors to test nitrites, glucose, protein, and pH for possible infections or disease. AI might be used to spot biomarkers that would indicate a potential change in health status before symptoms appear. Technology embedded in a bathroom mirror might be able to distinguish a mole from melanoma.
We are already seeing advancements in the ability to detect the early stages of cancer. A liquid biopsy, for example, can help identify cancer cells by testing a sample of blood. At a 2019 conference, California-based Grail presented data that it said confirms its ability to detect early-stage cancer with a single blood test. The company said it detected strong signals for 12 cancers with a 99 percent specificity rate.6
Nutrition and microbiome: Everyone has a unique microbiome, and researchers are just beginning to understand the relationship between these tiny organisms and how they influence mental and physical health. In 2018, the global human microbiome market was valued at US$351.81 million,7 and over 100 companies are investing in analyzing data related to the microbiome.8
The estimated 38 trillion microorganisms that exist within our bodies can have an impact on physical and mental health, which gives new meaning to the phrase “you are what you eat.” A better understanding of the microbiome could lead to more effective ways to prevent disease. Microbes found in the guts of children, for example, could be related to childhood diseases, such as Type 1 diabetes.9 Recent research also suggests that the microbiome could influence how susceptible a patient might be to certain types of cancer, or how a patient is likely to respond to immunotherapy.10
Considerations:Our understanding of what drives disease is continually evolving, and some diseases don’t have a clear underlying cause. As one interviewee pointed out, “There is no way to determine why some people develop rheumatoid arthritis and some people don’t.” But the groundwork is already being laid for a world with a greater ability to detect and prevent disease. Diagnostics, and their resulting data, are likely to become powerful tools going forward. Advanced analytics of data collected from diagnostics, combined with patient health records, could help identify patterns related to the causes and early markers of disease. Pharma companies should learn to leverage the insights that come from these technologies to develop pathways and treatments for early intervention.
Force no. 2: Personalized or customized treatment
The way individuals express disease and respond to treatment varies greatly. The vast majority of patients may not receive the full potential benefit of drugs that they are treated with because we don’t yet know how to effectively stratify patient populations, one interviewee noted. A therapy that is effective for one patient, might be metabolized differently by another patient and never reach the optimal active concentration, he noted. Giving each patient a personalized dosage, or the optimal combination of drugs, could lead to better outcomes.
We define a customized treatment as a single therapy, or mix of therapies, that is selected, tailored, or developed to treat an individual. This requires leveraging data to identify the best drug treatment option (single or combination therapy), the right dosing, and possibly customizing treatment for an individual patient. Getting to this level of customization will likely require a wealth of data—either through the use of real-world evidence (RWE) to effectively target or repurpose existing treatments—or new clinical trial paradigms that help identify high responders and optimal dosing. Given the need for data, we envision that the early advances in personalized treatments will be seen with generic and late–life cycle medications, which have a wealth of RWE available to inform the stratification of the disease, tailored dosing, and tailored regimens.
Stratifying disease to target the best drug intervention: Advances in the understanding of biomarkers and genetic markers have helped researchers identify subpopulations within broader disease categories. Parkinson’s disease, for example, has a number of clear genetic subsets and various mutations. It’s actually a set of discrete diseases, and some variations of Parkinson’s look different than others. In the future, we will likely be able to identify increasingly smaller subsets of patients based on genetic lesions, differences in protein expression, and the microbiome. Biopharma companies might be able to develop or target therapies to the unique characteristics of each subpopulation.
Tailored dosing: Predictive analytics and the ability to analyze longitudinal and integrated data sets for diverse patient populations could help biopharma companies determine optimal dosing levels for patients, who is most likely to respond, and under what conditions. This data could help companies develop customized treatment regimens for specific types of patients.
For example, data on how a patient metabolizes a drug could be used to ensure he/she gets the precise dose that will optimize effectiveness and minimize toxicity. The concept of pharmacogenomics has been around for a long time, but little progress has been made when it comes to using this information to determine effective dosing. Systematic evaluation of how patients metabolize drugs by evaluating the expression of liver enzymes (CYP450) or kidney function could enable more optimal dosing to get patients into therapeutic ranges. One interviewee said, “Pharmacogenomic data will become part of the electronic medical record.”
Tailored drug regimens: Clinicians of the future might be able to examine a range of biomarkers and genetic information—as well as clinical and behavioral digital health data—to determine the appropriate drug combinations for a patient. This could be similar to how doctors are now able to sequence tumors, identify mutations, and match the appropriate therapy in cancer patients.
Further, additive manufacturing could lead to new ways of delivering drugs. Active pharmaceutical ingredients (APIs) could be combined at the point of care, allowing for customized therapies. One physician interviewee predicted, “We will be giving patients one pill with everything rather than a number of pills.” She noted that this a common patient pain point that physicians often don’t see. Case in point: In 2015, Spritam became the world’s first 3D-printed drug approved by the FDA and was used to treat epilepsy. Further, startups such as FabRX are aiming to provide personalized medicines through 3D printing. FabRX’s proprietary technology, Printlets, offers personalized dosages, polypills, chewable medicines, and fast-dissolving tablets.11
Considerations: A proliferation of real-world data sources could help health care stakeholders understand which drugs work best for which patients and under which circumstances. Biopharma companies should be at the forefront of understanding heterogenous patient populations. However, being at the forefront will likely require significant investments in data and analytics capabilities. Companies should consider new drug-development paradigms, such as master protocols, that enable the evaluation of drugs alone or in combination in specified patient subpopulations.12 This shift toward increasingly customized treatments could have a significant impact on the biopharma supply chain. Smaller-volume therapies could require new manufacturing capabilities—not just for branded manufacturers, but all generics (see the sidebar, “The future of generic drug manufacturing”).
The future of generic-drug manufacturing
About 90 percent of all prescription drug purchases are for generics, according to the IQVIA Institute. Unlike some high-cost therapies that treat a small portion of the population, the generic-drug business model is based entirely on high-volume treatments that target large populations. If more diseases are cured or prevented, the need for generic drugs that treat chronic diseases could be reduced significantly, which could disrupt the volume-based model. Increased focus on nutrition, for example, could mean fewer people have high cholesterol, which would translate to a smaller market for generic statins.
If the future of health emerges in the way we expect it will, we could see some consolidation among generic-drug manufacturers. Most could benefit from developing a business model that focuses on more customized treatments. Manufacturers that are able to leverage prescribing and outcomes data for generic drugs may be well-positioned to match patients to appropriate therapies. Further, investment in additive manufacturing capabilities and distribution channels could enable companies to manufacture customized treatment combinations, and tailored doses. Manufacturers might also partner with consumer-focused retailers to develop customized drug packaging and distribute their products directly to patients. Some manufacturers might forge partnerships directly with health systems or consortiums to supply them with products.
Force no. 3: Curative therapies
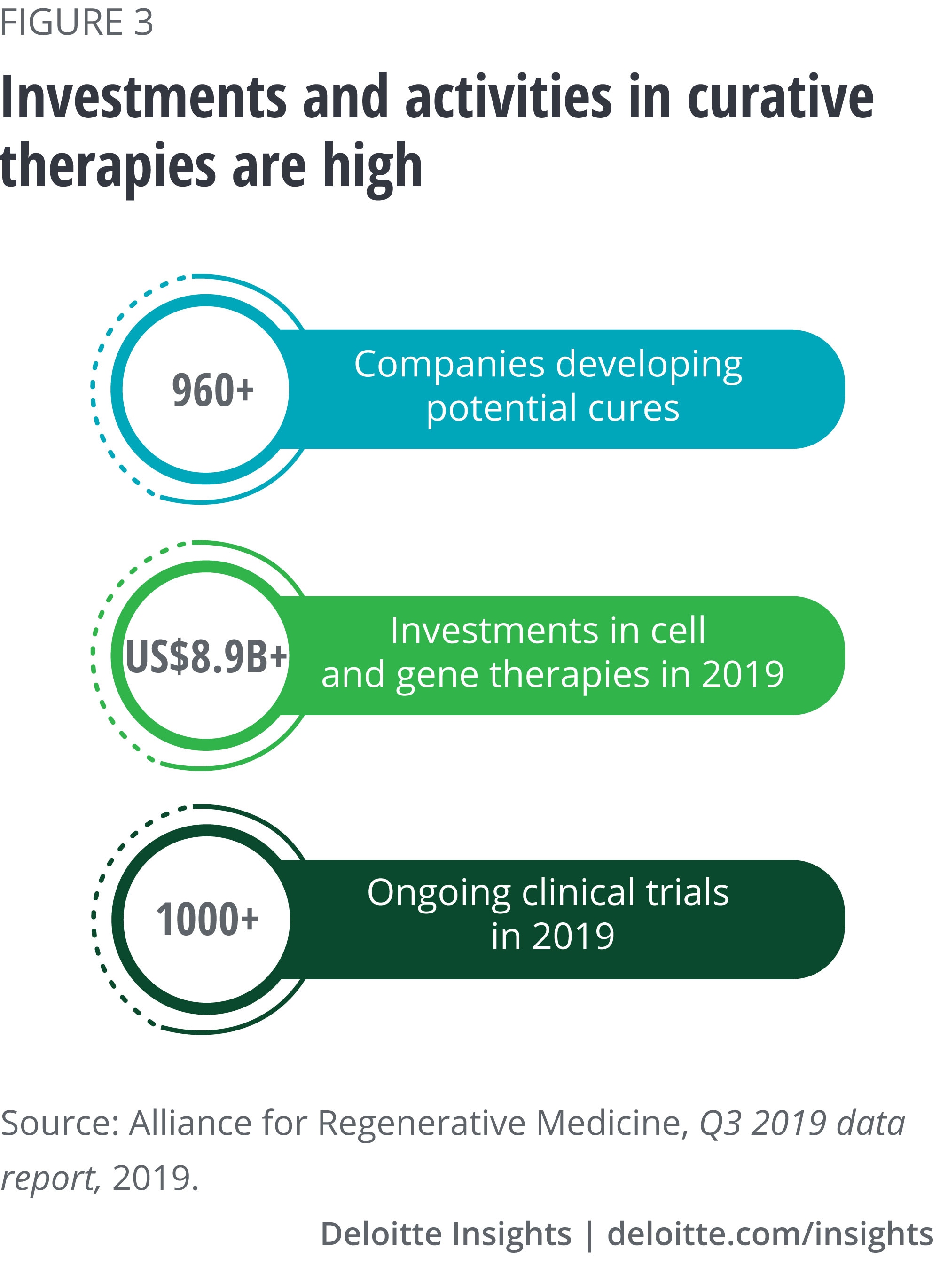
Curative therapies—time-limited treatments that remove symptoms of a disease through the permanent (or semipermanent) correction of the underlying condition—have the potential to reduce the incidence and prevalence of many diseases. Several of our interviewees predicted that diseases driven by single genetic mutations (e.g., certain cancers, sickle cell anemia, and some rare diseases) are likely to be among the first ones treated by curative therapies. At the end of 2019, more than 1,000 clinical trials were being executed worldwide for cell and gene therapies (see figure 3).13 These trials are targeting a breadth of diseases including cancer, musculoskeletal disorders, and neurodegenerative diseases. While biopharma is at the forefront of developing these treatments, keeping pace with a rapidly changing environment will likely require new business models.
Gene therapies: The first gene therapy in the United States was approved in 2019, and many more are in the pipeline. Most of these therapies target diseases driven by a single or a few genetic mutations. One interviewee said he expects to “see cures for diseases that are well-defined by genetic mutations based on better gene therapy.” Such diseases could include cystic fibrosis, sickle cell disease, fragile X syndrome, muscular dystrophy, and Huntington’s disease.
One academic researcher told us that we might be able to cure cancer subtypes that are caused by specific oncogenes passed down in families, such as BRCA. Gene replacement, for example, could replace prophylactic mastectomies. He cautioned that there are many safety concerns with this approach and said it could be difficult to replace the gene in all of a person’s cancer-causing cells. Diseases that are cell nonautonomous might be more attractive, he added.
Another interviewee told us that CRISPR has been used to destroy viruses and bacterial infections. He also said that CRISPR can be used to program bacteria to guard against other bacteria. For example, researchers at the Western University in London, Ontario, successfully used a CRISPR-associated enzyme called Cas9 to eliminate a species of Salmonella using E. coli bacteria. Scientists at the Broad Institute also recently published a study that demonstrated that another enzyme called Cas13 could be programmed to kill three different kinds of single-stranded RNA viruses.14 However, one CRISPR leader pointed out that gene editing might only address part of the problem for multifactor diseases. Drug therapy might still be required to manage other variables that drive disease expression.
Cell therapies: Adoptive cell transfer (ACT) therapy is driving research and development activity among many biopharma companies. The most publicized form of ACT (CAR-T) uses the patient’s own immune system to destroy cancer cells. Two therapies using this technology have been approved in the United States, and many more are in development.15 These therapies could treat previously difficult-to-treat late-stage cancers and have led to high rates of remission. As researchers reduce the cost of making and delivering these therapies, and reduce or better manage toxicities, they could move upstream in treatment plans to become the standard of care.
One interviewee suggested that applications of this technology could expand beyond oncology into autoimmune diseases. “We’ll better understand where the immune system has been programmed up and learn to program it down using CAR-T,” he said. He also suggested that we might be able to use gene and cell therapy to reprogram our bone marrow/immune system, but noted that it would be a “longer, harder road with weird speed bumps.”
Another interviewee pointed out that stem cells are the raw materials for cellular therapies, similar to bone marrow. During the next 20 years, biopharma companies might get close to the process of sourcing these raw materials and could consider using them to treat disease. “In the future, we might be able to extract bone marrow from a young patient, store it, and use it to cure an autoimmune illness that we know is likely to develop in that person as they reach their 40s,” he predicted. “This idea might be science fiction today, but 20 years from now, some pharmaceutical companies might have stem cell transplant centers.” Magenta Therapeutics is an example of a company that is working on this today.
Considerations: Curative therapies will likely require new business models that address the shift in cost from chronic treatment to a one-time treatment. The long-term health economic value of cures could be tremendous, but data will be needed to demonstrate value before widespread adoption can occur. Companies will likely need to develop sophisticated methods for monitoring post-treatment patients to help shape the value story. Questions about how the cost and benefits for a one-time curative treatment should be allocated need to be addressed. Lastly, companies might need to explore novel financing mechanisms to enable both access and affordability given that the short-duration treatment has the potential for a life time of benefit.
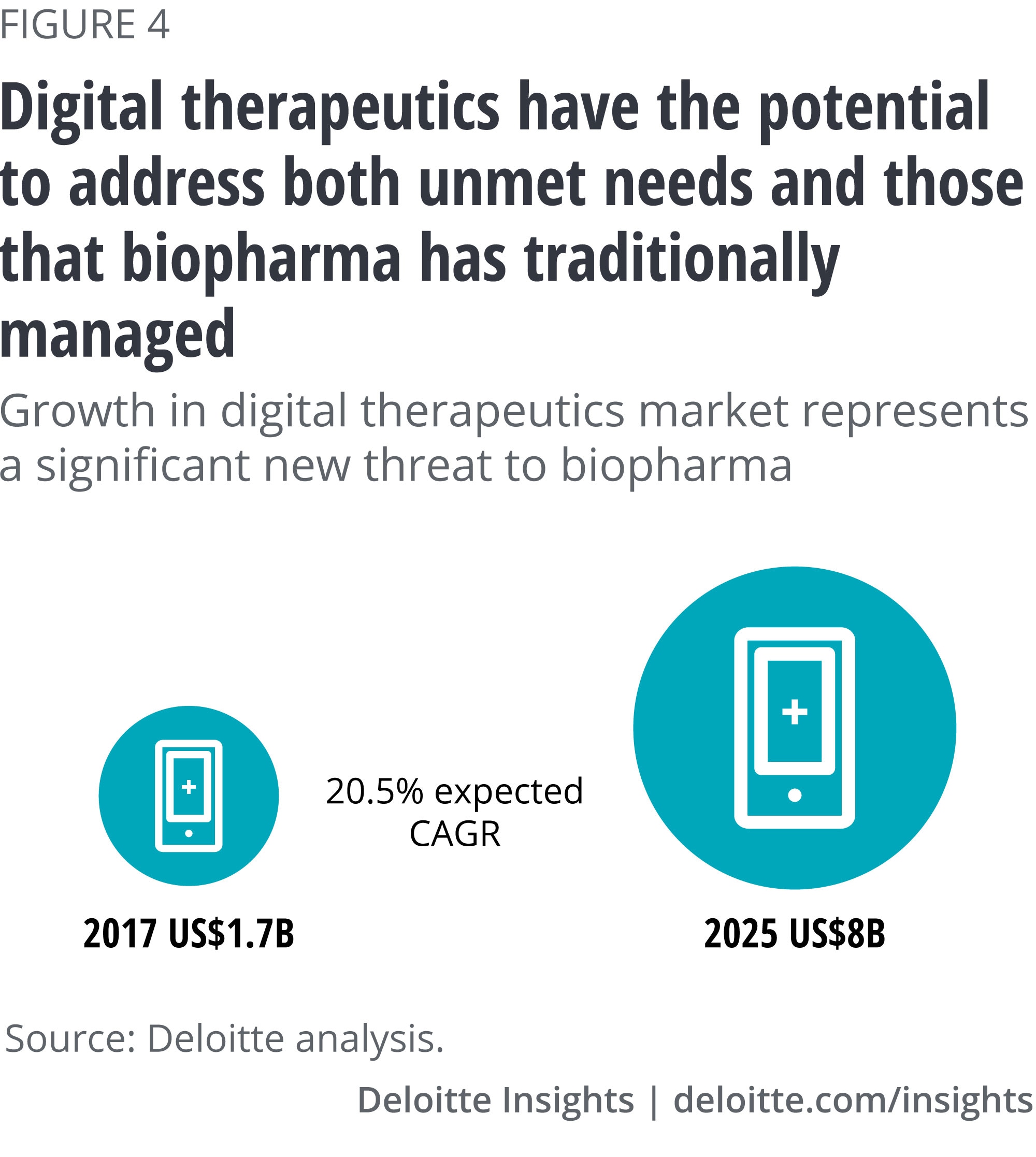
Force no. 4: Digital therapeutics
Digital therapeutics deliver evidence-based interventions to patients through software programs that help prevent, manage, or treat a medical disorder or disease. This technology could be a viable alternative to traditional pharmacologic treatments, or used in concert with medications, devices, or other therapies to optimize patient care and health outcomes. Digital therapeutics, for example, might use an app-based platform to target modifiable chronic diseases such as diabetes, depression, anxiety, and heart disease.
Digital therapeutics that help make drug treatment more effective: Some digital therapeutics are helping patients take a more holistic approach to managing their disease, including combining drug treatments with nondrug treatments. For example, diabetes digital programs combine a blood-glucose monitor with actionable insights and personalized coaching. Real-time tracking of drug therapy and symptoms allow doctors and coaches to intervene and modify therapy before symptoms escalate. Some of these digital interventions have been FDA-approved and health plans and pharmacy benefit managers (PBMs) are paying for them. Express Scripts, for example, has established a digital formulary that evaluates digital interventions based on clinical research, usability, and financial value.16 Livongo’s digital therapeutics for diabetes, prediabetes, and hypertension have received preferred formulary status (see the sidebar, “New digital therapeutics help patients manage their chronic diseases”).
Digital therapeutics that reduce the need for pharmaceutical intervention: Digital therapeutics are increasing access to medical providers, which could reduce the need for drug treatments. For example, many digital therapeutics rely on cognitive behavioral therapy (CBT), a psychological treatment that has been shown to improve patient outcomes across a range of mental health diseases. Virtual coaches, for example, can help patients modify behavior in the moment to reduce the symptoms of anxiety, depression, and insomnia. Sleepio, a digital treatment for insomnia that does not involve drug therapy, is one example that is covered by a PBM in lieu of pharmaceutical sleep agents. Some digital therapeutics are empowering patients to take charge of their symptoms for complex chronic diseases, including autoimmune diseases (see the sidebar, “New digital therapeutics help patients manage their chronic diseases”).
Consumers are becoming more proactive in managing their health
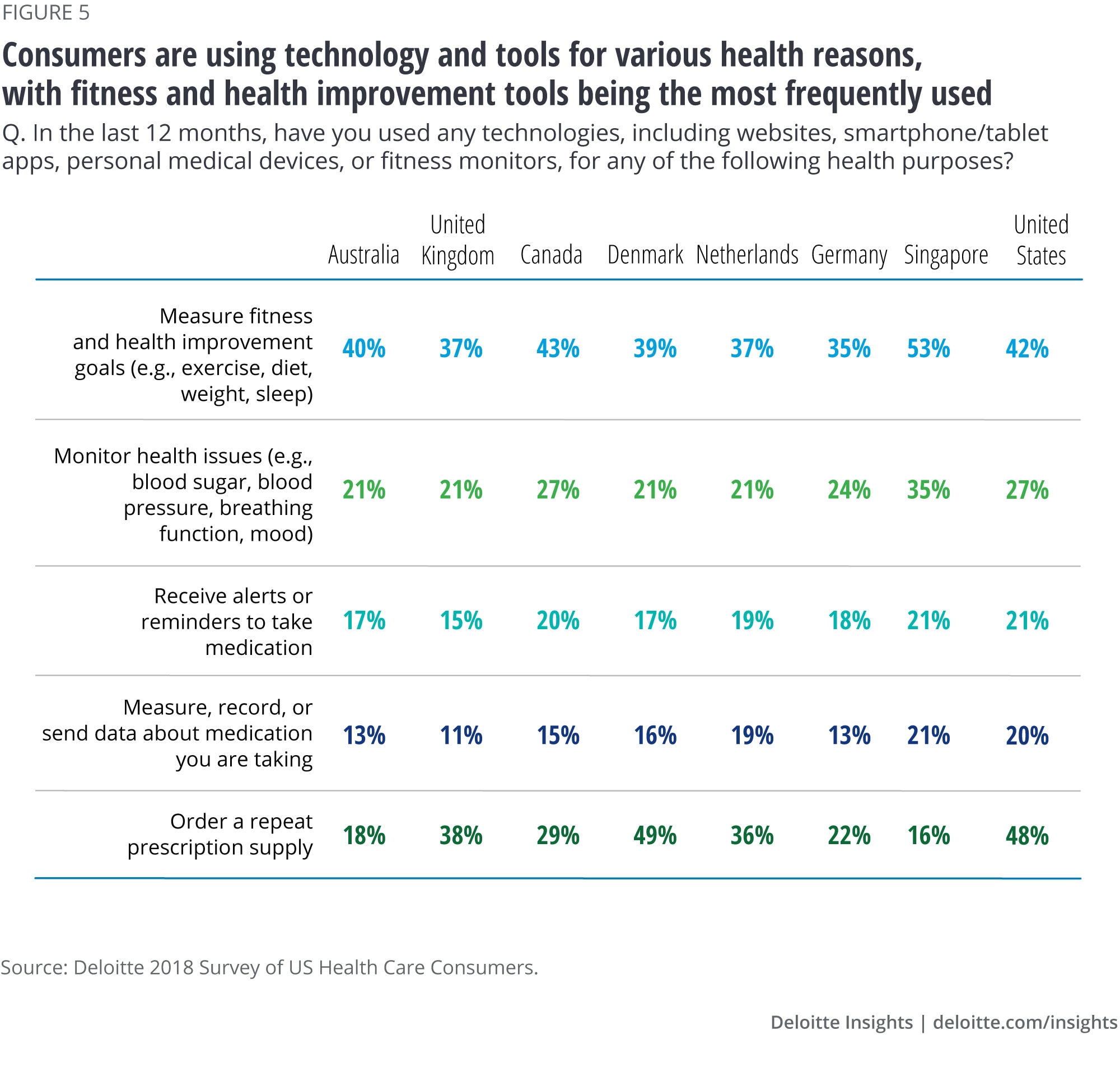
From exercise to nutrition to meditation, consumers are paying closer attention to their well-being. The proliferation of fitness trackers, smart watches, and other wearables that track activity and sleep has moved quickly from early adoption a few years ago to the mainstream. According to Deloitte’s 2019 Global Health Care Consumer Survey, 42 percent of US consumers rely on technology to measure their fitness levels and set health-improvement goals (figure 5).17 Patients are also increasingly interested in trying out homeopathic methods before turning to pharmaceuticals. Moreover, one of our interviewees suggested that physicians of the future might be writing prescriptions for healthy food18 and walks outside.19 This increased awareness of well-being, combined with digital therapeutics, could help consumers stay healthy.
Considerations: The future of digital therapeutics depends on user adoption, the ability to demonstrate impact, and optimization of pricing/reimbursement. Some interviewees were skeptical of the long-term adoption, or stickiness, of digital therapeutics. While some patients might try the technology, they might lose interest after a few days or weeks. Our interviewees also cautioned that higher socioeconomic classes are more likely than lower classes to use these technologies, which could further widen health disparities. Other interviewees countered that if the digital experience is user-friendly, includes a human touch when necessary, incorporates storytelling (rather than commands), and truly understands the motivations for each patient (whether that be more free time, better health, or something else), adoption will likely continue to grow. Non-negotiable, however, is that these technologies need to demonstrate results. One interviewee suggested that the adoption of health care apps will likely reach a tipping point once major health systems, health plans, and large pharmacies “prioritize digital therapeutics over prescriptions and pay for them.”
New digital therapeutics help patients manage their chronic diseases
Sleepio: Sleepio, a digital sleep improvement program, was founded in 2010. Using cognitive and behavioral therapy techniques to address sleep disorders, the app strives to be entertaining as well as tailored to each user. A 2012 paper published in the trade journal Sleep determined Sleepio to be more effective than a placebo and helped 76 percent of users achieve healthy sleep levels.20 In a 2018 JAMA Psychiatry paper, about 75 percent of Sleepio users saw an improvement in sleep after going through the company’s six-week program.21 These results spurred the United Kingdom’s NHS to pay for the use of the app.22
Livongo: Livongo, founded in 2008, is a digital health management app for chronic diseases. The platform focuses on multiple conditions, including diabetes, prediabetes, weight management, hypertension, and behavioral health. It aims to close gaps in care, provide refill deliveries, and personalize care through both its digital offering and access to real-time health specialists and coaches. In 2017, Livongo presented at the Annual American Diabetes Association’s Scientific Sessions showing that it decreased costs by 5.8 percent compared to non-Livongo users, helping save US$83 per participant per month.23
MyMee: The three-year-old digital care program focuses on chronic autoimmune disorders. It allows users to input and track their daily activities, and then make sense of any patterns in the data that might be impacting their health. It also connects users with a health coach. Anecdotal stories on MyMee’s website highlight individuals who were able to learn about and understand the triggers that are negatively impacting their bodies. In response, they were able to change their diets and lifestyle habits to avoid those triggers.
Force no. 5: Precision intervention
More sophisticated medical technology might enable earlier interventions and more effective procedures that reduce or even eliminate the need for pharmaceutical management. This includes advances in robotic surgery, nanotechnology, and tissue engineering. Some of our interviewees suggested that as these technologies become more sophisticated, they could lead to dramatically improved outcomes in cancer, infectious disease, inflammatory conditions, and chronic pain.
Robotic surgery: Historically, the scope of surgeries has been limited by the clumsiness of human hands, requiring drug therapy to manage the remaining disease. Robotic surgery is now the standard of care for many procedures, and medtech companies are investing in technology to advance capabilities including integrating them with augmented/virtual reality, AI, advanced analytics, and other emerging technologies. In the future, previously inoperable sites might be reachable, which could increase the efficacy of these platforms. For example, cancer patients who have hard-to-reach tumors have historically been treated with systemic therapies and radiation. Advances in robotic surgery have made it possible to remove a tumor wrapped around the spinal cord, for example, and eliminate or reduce the need for chemotherapy.
Nanotechnology: Microscopic nanotechnology particles have the potential to enter diseased tissues and deliver more targeted and precise medical interventions. One of our interviewees said, “We will have an active nanomechanical system that can go through and do different things in the body, like replace tissues and cell types.” Case in point: A team of scientists at the University of California, San Diego, created “nanosponges” that can go through the body and remove toxins from bacterial pathogens and inflammatory conditions.24 Nanotechnology might even evolve to include living cells. Researchers at the University of Vermont and Tufts University have designed organic robots by joining together specific types of stem cells taken from a well-studied species of African frog, Xenopus laevis. They suggest that bots derived in a similar manner, using the person’s own cells, could be injected into the bloodstream to remove plaque from artery walls.25
3D printing and tissue engineering: Manufacturers and clinicians could use 3D printing to create highly customized, low-cost medical technology products that are tailored to each patient’s unique physiology. Everything from prosthetics to skin for burn victims to organs to implants (dental and orthopedic) can be produced through 3D printing. In some applications, 3D printing offers solutions where none existed. For example, airway splints for babies with tracheobronchomalacia (a rare condition where the tracheal or windpipe cartilage is soft) can be created by a 3D printer.26
Tissue engineering—in combination with additive manufacturing—could be used to restore damaged tissues. One interviewee noted that we’d likely start there, and then we’d harvest organs. He suggested that at some point in the future, patients might be presented with multiple options to treat tissue or organ dysfunction. “We’ll be able to fix it, regenerate tissue, or grow replacements, perhaps through xenotransplantation,” he predicted. Advances in this technology could fix chronic diseases, eliminating the need for ongoing drug therapy. Case in point: Researchers at Cincinnati Children’s Hospital tissue-engineered human pancreatic islets in a laboratory. The engineered tissue develops a circulatory system, secretes hormones such as insulin, and has successfully treated sudden-onset Type 1 diabetes in mice.27
Considerations: These technologies could be highly disruptive to biopharmaceutical companies by eliminating the need for drug therapy. These interventions might be poised to reduce the need for major classes of drugs such as chemotherapy, insulin, and drugs for inflammatory conditions. Biopharma companies working in impacted disease areas should consider adopting some of these technologies, or risk operating in a much smaller market in the future.
Recommendations for biopharma
The five forces described above should push biopharma companies to ask themselves some hard questions about the markets they are in and how the threat of disruption could impact them. The current model of selling therapies that treat symptoms or mitigate the progression of chronic diseases is no longer viable. Sales volumes for drugs across disease areas are likely to decline due to more effective prevention, greater stratification of disease, better tailoring of drug regimens for patients, an increase in curative therapies, behavioral intervention, and advanced medical procedures.
In the face of these five forces, biopharma companies should ask themselves:
- How will each of these five forces disrupt our current business model?
- Where do we see new opportunities? What are the potential threats to our current business? How might these unfold over time?
- To what extent do we want to play in these emerging areas?
- How can we address these disruptive threats now? What capabilities are needed to enter emerging areas? What are some no-regret moves that we can make (e.g., potential partnerships)?
- How can we continue to monitor the landscape for future disruption?
Conclusion
By 2040, some diseases will be prevented, cured, or managed with nonpharmacological interventions. If that vision is correct, we could have fewer people with chronic diseases and less need for therapies that treat those conditions. As a result, what has historically been in-bounds for the pharma sector—such as the treatment of chronic diseases—could erode. “If pharma wants to survive, they should broaden themselves significantly,” said one former executive of a global pharmaceutical company.
When we look back on the sector 20 years from now, we may describe our current approach to disease and treatments as crude. Pharmaceutical companies that are able to reimagine their traditional business model may be most likely to succeed in a future built around prevention, early detection, and personalized therapies.
© 2021. See Terms of Use for more information.
Learn more about health care
-
Health care Collection
-
Digital health technology Article5 years ago
-
Virtual health care Video
-
Predictive analytics in health care Article5 years ago
-
Showing cybersecurity's value to health care leadership Article5 years ago
-
Creating a treasure trove of data for health plans Article5 years ago