Cell and gene therapies Delivering scientific innovation requires operating model innovation
26 minute read
17 April 2020
 Hussain Mooraj United States
Hussain Mooraj United States Omkar Kawalekar United States
Omkar Kawalekar United States Leena Gupta United States
Leena Gupta United States Sonal Shah United States
Sonal Shah United States
Potential disruption by cell and gene therapies calls for an overhaul of current operating models, with a focus on organizational structure, business processes, digital capabilities, risk tolerance, and reimbursement.
Executive summary
Cell and gene therapies (CGTs) are the next wave of therapeutic innovation in the life sciences industry; however, the capabilities to develop, manufacture, order, deliver, and get paid for these highly customized therapies are still nascent. Life sciences companies should design and build new operating models to realize the promise of CGTs.
Learn more
Explore the Life sciences collection
Learn about Deloitte’s services
Go straight to smart. Get the Deloitte Insights app.
Nineteen cross-functional leaders we interviewed along the CGT value chain (including manufacturers, treatment centers, and other partners) told us that new ways of thinking and operating are needed for companies to successfully bring CGTs to scale. However, there is no agreement about the best approach, given that there are no guides for what “good” or “best” looks like. As several of our interviewees rightly said, “We’re building the plane as we’re flying it.”
We synthesized their insights into five key elements of the operating model these organizations are trying to solve for and provide some thinking on where solutions may lie:
Organizing for success: CGTs call for the breakdown of traditional functional silos for small and large molecule development and commercialization. Some questions leaders are grappling with revolve around whether to perform activities internally or to outsource, centralize or decentralize, and whether to invest capital in capabilities that are not yet available in the market. The implications of these decisions can be long-lasting and difficult to change. While solving these issues, companies should consider factors such as the nature of the therapy, access to capital, existence of external capabilities, and ability to deliver the product quickly.
Optimizing business processes: CGT companies are intricately involved in both upstream and downstream activities related to making and delivering their products. These range from sourcing raw material from patients to tracking products until they reach the patient safely. Hence, a well-coordinated value chain with simultaneous input from all functions becomes inevitable.
Building digital capabilities: CGT companies typically require sophisticated digital tools, which are often being built from the ground up, to track biological material, critical patient information, and financial flow along the patient journey. The leaders interviewed are focused on figuring out which tools can be leveraged to enhance the clinician and patient experience and to support the generation of long-term evidence of efficacy and safety.
Embracing a culture of risk tolerance: Leaders across the value chain emphasized the importance of flexibility, risk tolerance, and rapid decision-making capabilities in CGT company culture. Obstacles and delays that would be considered failures in traditional biopharma are often inevitable in CGTs. Large biopharma companies that acquire CGT companies may need to consider ring-fencing them to maintain processes and allow some level of autonomy.
Experimenting with new payment models: Early clinical data for CGTs is promising, often enabling life-extending or life-altering benefits. While the value can be tremendous, it comes at a high price and there is uncertainty around the durability of response. CGT companies and payers are considering value-based contracts and alternative financing mechanisms to pay for these therapies.
Progress on developing infrastructure to support the delivery of CGTs has been slow, but our interviewees are optimistic and committed to the mission. They believe that companies should distinguish between areas of competitive advantage and opportunities for industrywide standardization and consolidation, where consortiums could help drive progress. Designing a value chain for CGTs could be a critical path to delivering these scientific breakthroughs to patients whose lives depend on them.
Introduction
Cell and gene therapies (CGTs) are poised to disrupt the biopharma industry1 in a way that hasn’t been seen in years. In 2018, investors committed over US$13 billion globally to advanced therapies, including cell, gene, and gene-modified cell therapy—signaling increasing confidence and enthusiasm for these therapies and their ability to treat diseases that have traditionally been difficult or impossible to treat. With more than 900 companies globally focused on such advanced therapies, and over 1,000 cell and/or gene therapy clinical trials currently underway, the industry could see a tsunami of approvals—as many as 10 to 20 new therapies per year—starting in 2025.2 And this will likely only accelerate with time.
Investment is flowing into companies of varying maturity, ranging from startups to major acquisitions by large biopharma companies. In 2019, 19 M&A deals worth over US$156 billion were completed (figure 1).3 The largest of these was Bristol-Myers Squibb’s US$74 billion acquisition of Celgene and its pipeline of multiple cell therapy assets to bolster its participation in oncology treatments.4 This represents a significant increase since 2015, when M&A activities in CGT totaled about US$4 billion.5

Despite the investment in and excitement about CGTs, their novel mechanisms of action, and their potential curative capabilities, the market is not yet ready for adoption at scale. Some of the most important challenges to commercializing CGTs are:
Complex manufacturing and distribution: CGTs are challenging, risky, and expensive to manufacture due to the lack of commercial scale of the technologies involved.
Atypical demand and impact on capability requirements: As CGTs are a third-line therapy in most cases, the patient pool can be hard to predict. Additionally, individualized therapies are made to order, in contrast to the typical made-to-stock model in traditional biopharma, making demand and capacity forecasting complex.
Complex administration and management: Serious adverse events such as cytokine release syndrome (CRS) and neurotoxicity are possible with CGTs. This requires advanced administration protocols, additional training, and specialized clinical resources to manage patients and for short- and long-term monitoring.
Sticker shock and outdated payment models: CGT therapies can be more expensive than traditional therapies and are offered as single-dose treatments with long-term clinical value. Even cures that are demonstrably cost-effective can face challenges if it takes inordinately long for payers to recover their costs.
Biopharma companies’ traditional business and operating models are often not optimal for bringing to market highly customized products like CGTs, which typically require precise material sourcing, tracking, and delivery. Despite the strong interest in and the promise of CGTs, companies have not yet defined, much less built, infrastructure that supports their manufacture and delivery at scale. The life sciences community should focus on building a critically important value chain that can bring these therapies to market at scale (see sidebar, “Shifting the model from supply chain to value chain for CGT”).
Shifting the model from supply chain to a value chain for CGT
Supply chain refers to the integration of all activities involved in the process of sourcing, procurement, conversion, and logistics. On the other hand, value chain is the series of business operations in which utility is added to the goods and services offered by the firm to enhance customer value.6 Our definition of customers includes treatment sites, patients, and other relevant stakeholders. Figure 2 depicts the typical, made-to-order CGT value chain.

We spoke to experts across the CGT value chain, including Deloitte practitioners, for perspectives on the current state of cell and gene therapies and an understanding of the major barriers to realizing their full potential (see sidebar, “Methodology”).
Methodology
The Deloitte Center for Health Solutions interviewed 20 executives from cell and gene therapy companies, partners, treatment sites, and academic institutions in the United States and globally in late 2019 and early 2020. Company executives came from a variety of functional areas including supply chain, commercial, technology, finance, manufacturing, and patient operations at organizations in varying stages of business maturity. Our findings were supplemented by Deloitte practitioners with extensive experience in this industry. We sought to understand the unique operating model challenges for CGT companies as well as their critical ecosystem partners.
Cell and gene therapy operating models: Getting it right the first time
Interviewees said that even as CGTs are evolving rapidly, so are the processes and technologies that support their development and delivery. As a result, companies are dealing with several challenges—some of which are to be expected for any early-stage technology, and some unique to CGTs.
Notably, interviewees identified two major “moments of truth” when treating patients with CGTs:
Was the product available and ready to be used, on time, and at the right location when the patient needed it?
Did the therapy bring about the outcomes that were expected and desired by the patient and the physician?
Traditionally, when seeking regulatory approval for a drug, companies must prove efficacy and safety. But for CGTs, our interviewees told us that the biggest challenge is making sure the product being given to patients is the same as the one tested in clinical trials. Regulators have expressed this same sentiment—that typically new drug reviews focus 80 percent on clinical, and 20 percent on Chemistry, Manufacturing, and Controls; for CGTs that ratio is reversed.7 Companies may thus need to prioritize resources and investments differently.
CGT development and delivery are occurring within both large biopharma companies as well as small, early-stage companies. Larger biopharma companies must make tough decisions about the degree of integration of CGT acquisitions. Smaller CGT companies should invest in basic infrastructure at the same time as they are developing cell and gene therapy-specific processes and technologies. Both types of organizations are looking to emerging external partners in the CGT ecosystem to access new capabilities. We heard from our interviewees about opportunities for companies to come together to standardize several processes across the industry.
In this article, we describe some of the unique operating model challenges that CGT companies face and discuss approaches to addressing these challenges and opportunities for standardization versus competitive differentiation.
Organizing for success
Developing a patient-centered CGT value chain is a new competency requiring a whole new way of organizing and operating. Further, many of the capabilities required may not even exist yet:
Functional roles are being newly defined: CGTs are highly customized products requiring precise material sourcing, manufacturing, tracking, and delivery. The CGT model is centered around the patient and requires close integration of the supply chain and operational elements of an organization with the commercial elements, underpinned by unique technology.8 Almost all interviewees talked about functional boundaries breaking down. The traditional, largely linear process involving a series of handoffs, starting with early development and ending with commercial launch, will not suffice for CGTs. Nearly all key decisions require simultaneous input from quality assurance, quality control, regulatory, process development, manufacturing, clinical/commercial, and supply chain functions. New functional roles are being defined, such as patient operations, that deal with the delicate and nuanced interface between the manufacturing process, the treatment site, and the patient.
Distribution and the role of affiliates: In CGT, the function of regional affiliates, which traditionally play a fundamental role in the marketing, distribution, and payment of drugs sold in specific countries, is uncertain. Interviewees said, that given the customized nature of cell therapies, the small volumes, and the short turnaround times, regional affiliates are unlikely to play a role in distribution. Instead, direct-to-patient distribution will likely be centralized through manufacturing facilities or regional hubs.
However, regional affiliates could possibly continue to play a role in offering specific services to local markets; for example, in certifying and engaging treatment sites or tracking patient outcomes after the delivery of therapy. But one interviewee suggested that even these services may not make sense at a regional level because of the small volumes of therapies being delivered. For patients in remote areas with no qualified treatment sites, manufacturers should consider how to interface with them, support their travel, and plan for distribution and administration.
Build vs. partner: CGT companies are also grappling with decisions on whether to build internal infrastructure—dedicated technology systems, manufacturing capabilities, etc.—or to rely on external partners. These investments may be difficult to justify or predict given the current knowledge of efficacy and durability. This has implications across several value chain components:
R&D: CGT companies often rely on academic researchers to source innovation. In fact, the first few approved CAR-T and in vivo gene therapy products were all first tested in humans by academic institutions. One researcher we interviewed expressed interest in partnering with CGT companies earlier in the product development process. The goal would be to invest more time and money into preclinical studies, reduce attrition of products that are not likely to succeed in human trials, and preserve resources for products that show the most promise in the lab. He suggested that companies should partner more with academic labs to advance the delivery of therapies to targeted tumor types and make them more effective.
Material sourcing, tracking, and delivery: For autologous CGTs, the patient is the source of raw materials. This means there will be inherent variability in sourced materials, and thus requires more flexible process controls than any other type of product. External partners can be vital to supporting the sourcing, tracking, and delivery of cells for autologous therapies. CGT-specialized vendors have developed core competencies in parts of the supply chain that are critical for or specific to CGTs (e.g., Be The Match Registry—apheresis and registries; TrakCel and Vineti—logistics tracking and management). Some interviewees emphasized that organizations experienced in apheresis collection could help drive standards and efficiencies in that part of the process.
The implications of source materials on the CGT value chain
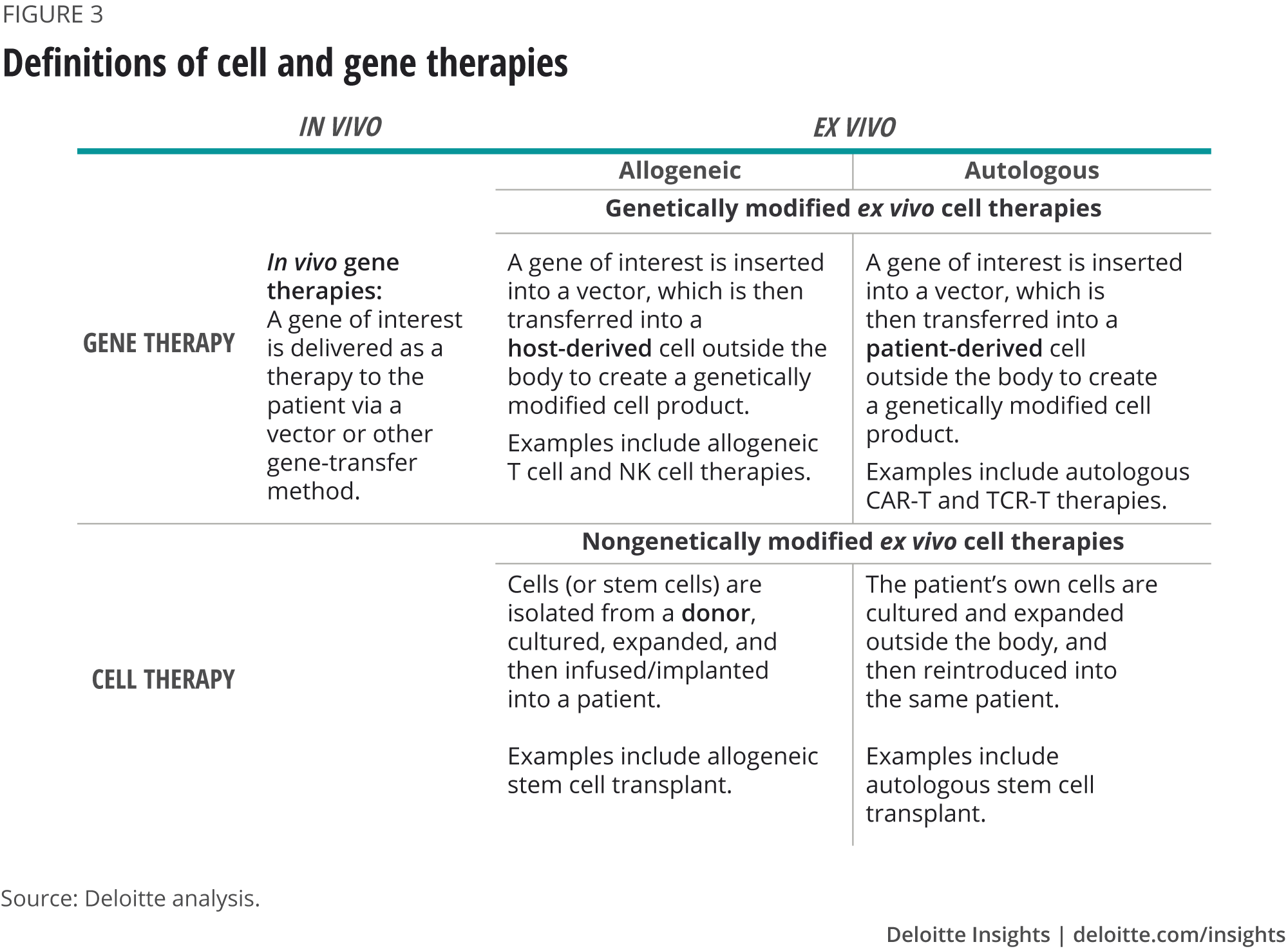
Based on the location of the genetic manipulation and source of raw material, CGTs can be broadly classified into two types—ex vivo and in vivo (see appendix for definitions).
Ex vivo therapies imply that cells or tissues are extracted from the human body and then manipulated outside before being injected back into a patient. Ex vivo CGTs can be further segmented into autologous and allogeneic, both of which require raw material to be sourced from a human subject. Autologous therapies require cells to be sourced from and returned to the same patient, whereas allogeneic therapies are delivered using material from any suitable donor. Autologous therapies are difficult to scale but in many cases are the preferred treatment route because of safety concerns, so many CGT companies are focused on building a value chain to support the manufacture of these therapies. Interviewees, however, stressed that the manufacturing process will differ greatly once allogeneic therapies become more viable.
In vivo therapies, on the other hand, facilitate genetic manipulation directly within the human body through the administration of a therapeutic product. These do not require raw material from human subjects, and instead rely on vectors for gene transfer; the most commonly used vectors are viral.
Successful commercialization of these products will likely require CGT companies to develop separate value chains where materials and related activities can be tracked according to their origin and destination as per the type of therapy.
Manufacturing: The decision on whether to invest in capital infrastructure is perhaps the most challenging and cost-prohibitive for CGT companies. These investments are large and have long-term implications for business operations. Most of the companies we interviewed were in preclinical and clinical stages and had not yet determined how and where to scale manufacturing. Interviewees pointed to several options ranging from a fully decentralized approach to a hub-and-spoke model. Ultimately, decisions on designing a manufacturing network will be grounded in key objectives including executing against predictability, stability, and fast turnaround times.
Leaders said factors influencing their decision on whether to invest capital or outsource include the nature of the therapy (allogeneic vs. autologous), the disease state and the volume of drug required, and the availability of external capacity and expertise. One interviewee pointed out that process decisions made in clinical stages determine what technologies they will have to rely on for commercial-scale operations.
Because of the significance of the capital investment required and the speed at which products must be launched, earlier-stage companies tend to rely more on contract development manufacturing organizations (CDMOs) than later-stage companies or those that had been acquired by large biopharma. However, this often means relinquishing full control of the manufacturing process to the CDMO with little oversight, which ties them to a third party regardless of performance. Some large biopharma companies are building in-house facilities. One company has set up a specific internal team to figure out how to commercialize and scale therapies. But the upfront costs with this option are high and there is a risk of being stuck with expensive, unused infrastructure in the event of product failure.
In response, hybrid models—where companies make smaller upfront investments for earlier-phase trials and then work with CDMOs for later-phase trials—have begun to emerge. Some investors are now offering “equity for manufacturing capacity,” or a shared-service model for early-stage companies to access services such as process development and regulatory and quality support.
The decisions leaders should make in developing a service delivery model for CGT are many, but one thing is clear: It’s important to get it right the first time. Changes to the model are difficult, expensive, and slow to make. One interviewee suggested that companies are almost stuck with the decisions they make early in the life cycle of developing a CGT asset.
Optimizing business processes
The process of developing, manufacturing, and delivering CGTs is complex, resource-intensive, and challenging. Unlike traditional biopharma, CGT companies need to be involved in both upstream and downstream processing. While traditional biopharma companies make drugs and distribute them through conventional channels, CGT companies must follow their products through the manufacturing process, starting from raw material collection, all the way through to delivery to the patient (see sidebar, “Chain of custody and chain of identity”). This process can be extremely resource-intensive and requires specialized knowledge and technology support.
Some of the processes involved in this chain could benefit from standardization, while others offer opportunities for differentiation and competitive advantage. For example, provider interviewees mentioned marked differences in customer engagement and services between two manufacturers of commercialized products they had administered. At the same time, they expressed frustration with having to use two different systems to effectively achieve similar process steps. Other potential areas for improvement include:
Specimen collection: For autologous therapies, specimens must be sourced from the patient, shipped to a manufacturing facility, and then returned to the patient treatment center. Interviewees said there is a sense of urgency in this phase, where treatment teams expect the “vein-to-vein” time to be as low as possible (in some cases, as little as two weeks). This must be done while maintaining chain of custody and chain of identity and meeting regulatory requirements. For allogeneic therapies, the source material is not derived from an individual patient, but from a healthy donor. However, interviewees said there seems to be a shortage of donor cells overall, and the FDA has only made the screening process for donors more selective.
Despite the complexity, many CGT companies prefer to do their own specimen collection for quality control. But manufacturer operations may conflict with the standard operating procedures of clinical sites. Treatment centers reported feeling overwhelmed by external entities and their multiple approaches to collection. Once specimens are collected, the window to manufacture the therapy and deliver it to the patient is small. Additional challenges in the process include variable, and often low, yields, maintenance of the cold chain during transport, and the short shelf life and overall delicate nature of the biological material.
While the requirements for specimen handling once it is extracted from a patient are variable, there may be opportunities to standardize specimen collection and develop best practices between apheresis operations experts, clinical sites, and manufacturers.
Where’s my stuff? Chain of identity and chain of custody
Chain of identity (COI) allows traceability of a drug back to the original donor and its lineage to its intended recipient. The raw material (tissue or cells) and the resulting drug product are associated with the donor’s unique identifiers throughout the entire process, from collection to manufacturing to treatment and post-treatment monitoring. For instance, as part of the COI, an autologous donor’s patient number should be associated with their unique donation number and manufacturing batch number.
Chain of custody (COC) allows traceability of a product through the value chain, from material collection to product administration. This includes data points such as staff handling information, temperature and storage conditions, actions performed, and the associated location, date, and time of those actions.
Chain of identity and chain of custody in CGT are distinct from traditional biopharma product serialization, since the patient is a key element of the manufacturing and delivery process. It is vital for the right patient to receive the right therapy to avoid safety issues such as product rejection. Once the therapy is administered, the COI/COC become part of the health system and provide complete traceability over the patient’s lifetime and ensure patient safety for multiple products and doses.
Labeling: Our interviewees flagged the physical labeling of CGTs as a difficult and time-consuming task with significant implications for operations and safety. They listed a variety of troubling issues across manufacturers, ranging from illegible, handwritten labels to nonstandard ones that include extraneous and confusing information. To compound the problem, most manufacturers have supplied their customers with printers that are only capable of printing labels for their specific product(s). Clinical sites working with multiple vendors must house multiple printers, which not only takes up physical space, but also requires operators to be trained on how to use them based on each manufacturer’s unique operating procedure.
There is an opportunity for manufacturers to standardize labeling through a consensus on key data inputs and outputs; all interviewees familiar with this issue are willing to participate in such an exercise. In response to the demand for industry and clinician harmonization, Deloitte started the Industry Working Group (IWG) Labeling Initiative. The IWG brings together CGT industry experts, including top pharma executives, clinicians, apheresis nurses, quality assurance staff, and regulatory advisors, as well as technology solution company executives. In close collaboration with the Standards Coordination Body, the group has put together a proposal for standardizing the minimum required elements for labeling of the apheresis product for autologous cell therapy manufacturing. These elements include label size, material, layout, and minimum data requirements, among others.
Therapy delivery: CGTtreatment sites must not only be audited and undergo Foundation for the Accreditation of Cellular Therapy (FACT) accreditation, but must also train personnel on drug profiles, procedures for sample collection and shipment, and portals to interact with the manufacturer. This is a time-consuming process for the clinical staff, despite similarities across products and manufacturers. Interviewees at treatment sites said these activities could be improved and standardized and that they would be more willing to work with companies that can make the training and onboarding experience as easy as possible.
The location of the patient at the time of treatment is another variable. In some cases, patients are admitted and treated as inpatients; in others, they are treated and monitored as outpatients but must have suitable housing nearby to deal with issues that may arise. While some treatment centers can arrange this, others cannot. This has implications for both staffing and patient management. Some CGT companies noted that offering hospital and patient support services is a potential differentiator. Outside of support services, however, CGT companies should consider staying away from the treatment process. One leader said that it would be “foolish” for CGT companies to think they could do a better job of it than treatment centers.
In addition to administering CGTs, moving the therapy itself to the treatment site can be complicated. Companies rely on third-party logistics companies to move specimens to and from treatment sites. Our interviewees described the variety of services they use to ensure the safe transportation of live material, but noted that the number of service providers and the service footprint would have to increase drastically once CGTs are produced at scale. Some CGT companies pointed out that physical delivery and delivery-related customer service are potential areas for innovation for third-party logistics vendors.
Patient tracking and monitoring: The FDA requires providers to follow up with patients who receive CGTs for 15 years after treatment—much longer than traditional biologics.9 Interviewees stressed that this is difficult in the current environment. Patients often travel long distances, sometimes internationally, to receive treatment, and additional issues can arise when patients switch providers or insurance plans.
The generation of long-term, real-world evidence is not only an FDA mandate, but could also be a source of competitive advantage for CGT companies. The data could also be used as input for product and process development. Companies that can demonstrate durability of treatment response are more likely to gain and retain market access and market share. Therefore, CGT companies should consider investments toward patient data collection a top priority.
Navigating time-sensitive CGTs during the COVID-19 outbreak
While life sciences companies quickly work to develop treatments and vaccines for the novel coronavirus (COVID-19), those that manufacture and deliver CGTs are feeling the impact of the global economic disruption the virus is causing. With restrictions on movement, companies are grappling with how to move time-sensitive CGT manufactured materials. For example, Novartis typically uses passenger aircraft to transport its CAR-T therapy, Kymriah, from Europe to the United States, but wasn’t able to due to a ban on travel between the two continents. Novartis said in a statement that they had “secured alternate options” of shipment in order to avoid delays that could affect treatment.10
Meanwhile, CGT company partner organization Be the Match says it has been monitoring the potential disruption to the cell therapy supply chain since the news of the outbreak in Wuhan, China, first began late in 2019, and checked in with partners around the globe to understand the true conditions on the ground. For an organization that depends on freedom of movement within and between countries, forging a plan to deal with many different scenarios was key. How could they ensure couriers could get from one place to another on time? What would happen if a courier became sick during a trip, and was quarantined as they were bringing product into the United States?11 These and many other questions had to be thought through and answered in order to minimize the disruption in delivering CGTs to the patients who need them.
Clinical trials for novel CGTs, along with other innovative treatments in biopharma, are also expected to slow down.12 The capacity to prepare and treat patients with experimental cell-based therapies in a hospital setting may be much more limited as hospital resources are prioritized for COVID-19 patients. This could be especially challenging for CGT startups that may struggle with access to capital to enable continued research efforts.
Building digital capabilities
Technology can be critical to the success of CGT. In fact, one interviewee thought of his company as being in the software business—in addition to CGT. Some technology applications are unique to CGTs and need to be built; others, such as back-office support services technology, can be leveraged from existing biopharma technology infrastructure. Specific CGT applications include those that can trace therapies through the make, order, and delivery process. Many CGT companies are interested in building tools that can digitally reflect the end-to-end patient journey, provide a differentiated and consistent end-user experience, and enable architecture to accommodate global regulations and market access requirements.
However, challenges exist in educating stakeholders, from leadership to clinical providers, on the unique needs of advanced therapies. Besides, standards are yet to be defined and most companies are building their own solutions. Some vendors are developing platforms and applications specific to CGT, but our interviewees suggested that these have not been universally adopted. This has important implications for both workflow management as well as evidence generation down the road.
Technology challenges are particularly significant for early-stage companies that are simultaneously building basic infrastructure and developing CGT-specific technologies. Some interviewees from acquired companies said they were happy to be absorbed into the parent company’s platforms for back-office functions, while others expressed frustration with conforming to a system that doesn’t suit their purpose. Further, a successful digital platform will likely need to be interoperable with generally accepted processes in health care and enable coordination among key stakeholders (manufacturers, providers, etc.). Independent companies noted that the opportunity to start from scratch was welcome, enabling them to use cloud technology and more agile approaches than those in the large biopharma environment.
While digital platforms are needed and will be useful, technology alone cannot cater to all user needs. Postinfusion monitoring and long-term follow up of patients may require solutions outside the digital realm.
Embracing risk
Our interviews showed that CGT organizations should be agile at this early stage of their evolution. However, acquisition by large biopharma companies could compromise agility, as the parent company takes over some decision rights and resource allocation. Many of our interviewees felt that bureaucracy and slow decision-making due to the size and scale of large biopharma can hinder progress.
In terms of talent, there is no pool of CGT executives and professionals to draw from and everyone is learning as they go. Risk tolerance, an ability to “roll up one’s sleeves,” make quick decisions, and work in the face of considerable ambiguity was at the top of almost all interviewees’ list of traits that a successful CGT professional should possess. An overall company culture that supports and, perhaps more importantly, understands the uncertainty of the CGT business and its associated setbacks is paramount to maintaining the passion to succeed.
Large biopharma companies may need to employ unique portfolio decision-making and resource allocation approaches for CGTs. Our interviewees noted that CGT teams operating as part of a larger portfolio can often be under-resourced, as their potential return on investment is often eclipsed by that of bigger products. To offset this impact of potential returns on funding decisions, large biopharma companies should consider longer-term returns on advancing a portfolio of products, rather than individual ones.
Interviewees were also of the opinion that in the case of acquisitions, immature businesses should be enabled, rather than absorbed, into an existing system to protect and nurture innovation. More established businesses are better able to handle a shift in operating practices imposed by an acquirer. Our interviewees also pointed out that there is often limited appreciation for the unique talent and capabilities within a CGT company even as retaining the right people and processes through a transaction is critical to preserving innovation and growth. In fact, from the perspective of both CGT companies and large biopharma, the most successful mergers have been those where core competencies were retained.
Experimenting with new payment models
Cell and gene therapies may promise a paradigm shift in medicine overall and immense value to patients, but the one-time cost associated with CGTs can cause sticker shock. Zolgensma, the spinal muscular atrophy (SMA) gene therapy treatment commercialized by Novartis, is priced at US$2.125 million, making it the world’s most expensive single-dose drug to date.13 The prices reflect the high cost and complexity of developing CGTs, as well as the significant value created for patients and their families. As more CGTs are approved for indications impacting bigger patient populations, costs could put a significant strain on health care budgets. One projection estimates approximately 350,000 patients will be treated with 30 to 60 CGT products by 2030.14
Initial clinical outcomes from CGTs are compelling, and the resulting benefits to patients, caregivers, employers, and society are tremendous. Patients can be cured of debilitating and life-threatening illnesses—gaining years of life, productivity, and avoidance of additional medical expenditure. However, these value drivers can be difficult to quantify. At the same time, the long-term durability of patient response is not yet known. Questions remain about who should pay for these therapies, who should own the risk, and who is liable for product failure.
The current health care system creates conflicting incentives in terms of who should pay for the value these therapies can provide. Insurers who pay for a one-time treatment may lose patients to another insurer, along with the benefit of having a healthy patient in their risk pool. At the same time, providers and patients are taking on significant financial risk by offering these therapies. Treatment centers are currently responsible for covering the cost of CGTs at the time of product delivery without knowing if the product will work. Payers don’t reimburse until later, once the therapy outcomes have been realized—and every instance of approval is individual, and payment can be insufficient—creating a huge financial risk for institutions (see sidebar, “Current reimbursement for CGTs is insufficient”). On the other hand, CGT companies worry that if treatment is not delivered exactly according to protocol, it could be ineffective.
Current reimbursement for CGTs is insufficient
Payers, including Medicare, are slow to cover CGT costs. Interviewees from treatment centers told us that Medicare’s payment for CAR-T therapies does not cover the entire cost, nor the treatment for the side effects of the therapy. Under the New Technology Add-on Payment (NTAP) program, Medicare covers 65 percent of the cost of CAR-T therapies, while hospitals cover the remaining 35 percent.15 In addition to the cost of the drug, hospitals seek payment for services surrounding the delivery of drugs, including apheresis, inpatient stays, and toxicity management, which are currently covered under the same DRG (diagnosis-related group) code as autologous bone marrow transplants. Treatment centers said they need to balance how many patients they treat under commercial and Medicare plans to remain financially viable. They also flagged that financial considerations come into play when deciding whether to offer patients commercially approved drugs or clinical drugs. In some cases, clinical-stage therapies can be more affordable for the patient as well as the institution.
CGT companies and insurers are exploring novel ways of addressing these challenges through value-based contracts (VBCs) or alternative financing mechanisms.
Value-based contracts: Some manufacturers have started to publicly communicate their intention to anchor payment to longer-term outcomes.16 However, interviewees told us many payers lack interest in such contracts because of complicated reimbursement mechanisms and the investments needed to support them (e.g., technology infrastructure to track clinical outcomes over time). Patients may be lost when they change physicians or health plans. Given the few approved therapies on the market, which target narrow indications and small patient populations, the cost of implementing VBCs may outweigh the possible benefits.
Alternative financing mechanisms: Some stakeholders have started to consider alternative financing mechanisms to address this challenge in the future. For example:
Cigna has announced its Embarc program, which allows covered beneficiaries under employer health plan sponsors to receive gene therapies for zero cost-sharing. Employers will contribute as little as US$1 a day for all enrolled beneficiaries.17
MIT’s NEW Drug Development ParadIGmS (NEWDIGS) initiative, Harvard Pilgrim, and other Massachusetts payers are collaborating to develop a “performance-based annuity” approach to paying for Zolgensma. The pilot would include an initial payment and subsequent annual payments thereafter, the price of which would be determined based on how patients respond to therapy over time.18
Manufacturers, payers, providers, employers, value assessors, and other stakeholders will need to answer some key questions as cell and gene therapies continue to come to market, including how to determine value and how to allocate costs and financial benefits. Some manufacturers we interviewed said they are focusing on quantifying the value directly to patients, based on the transformational health outcomes they achieve. One commercial leader told us that early engagement with payers and value assessors to understand how they define value is critical to incorporating the right endpoints into clinical and observational trials.
Next steps
Our CGT industry interviewees and colleagues in Deloitte’s next-generation therapies practice are optimistic about what the next 3–5 years will bring. In that time frame, we see several opportunities for improvement and innovation:
Pursue purpose-built organizational design: CGT companies should diverge from traditional biopharma structures and focus on building organizations suited to their needs and based on redefined functional roles, innovative distribution models, and partnership ecosystems; they should also implement more agile processes where possible. Risk tolerance should be incorporated as a cultural element. Large biopharma companies looking to expand their portfolio to include CGTs through acquisition should consider ring-fencing the acquired company’s operations and retaining as many key staff as possible.
Evolve the value chain: The CGT value chain is the determining factor for delivering on the two moments of truth discussed above (i.e., product availability at the critical time and achieving the desired outcome). Drug discovery and approvals will continue, but if manufacturing and/or therapy delivery is prohibitively difficult or expensive, patients will likely not benefit. Building a value chain around the patient that is both clinically connected and digitally enabled is vital. Investments should focus on:
Standardizing where necessary: While most interviewees acknowledged the many unknowns in the development of the CGT industry, they believe that standardizing some activities would be in everyone’s interest. For example, almost all clinical service providers we spoke to emphasized the need for coordination across inspecting and accrediting bodies to minimize disruption of their day-to-day activities. Companies should consider developing consortia and working groups to push for regulatory guidance and to tackle issues, such as labeling, to create a more harmonious experience for clinical customers.
Partnering when possible: In addition to industry consortia, network partnerships are important for aspects of the CGT value chain which may be too complex or costly for CGT companies to develop competencies in, including specimen collection and delivery logistics.
Differentiating on customer experience: Companies should look for differentiation in certain areas, particularly those related to the two moments of truth. This could include investments in enhanced technology and support services for treatment centers, as well as education of patients.
Deliver on digital: CGT companies should invest early in building a digital architecture that can support the patient journey. Data sourced from digital tools can provide a critical, competitive advantage by demonstrating that CGTs are delivering against promised health care outcomes in a timely manner. This evidence can further strengthen value propositions and help companies gain market access and market share.
Seek new sources of talent: CGT companies should consider looking at unconventional sources of talent, given the small network of professionals familiar with these technologies. It could be beneficial to look at provider systems as a source, especially at individuals with experience in stem cell transplant (or other complex procedures). Companies should also invest in retraining existing staff to meet the needs of CGTs. Aligning with industry bodies and educational institutions could be useful in nurturing and building a talent pool.
Collaborate to test new payment models: Manufacturers and payers should collaborate to determine the value of CGTs and define new payment models. Even though the investment may outweigh returns in the short run, the long-term payoff will be significant when more therapies come to market and treat significant numbers of patients.
In Deloitte’s view of the future of health, curative therapies such as CGTs are likely to be one of the major forces to disrupt biopharma.19 CGTs hold the promise of treating diseases in an entirely new way, or even curing those that until now have been debilitating or life-threatening. While it is important that CGT companies continue to innovate and push treatment technologies forward, it is now essential that they engage key stakeholders and build an infrastructure that can help bring these exciting advanced therapies to patients.
Appendix
Definitions of cell and gene therapies
Explore more
-
Intelligent drug discovery Article5 years ago
-
Intelligent clinical trials Article5 years ago
-
Drug and inpatient spending lines are crossing Article5 years ago
-
Striving to become more patient-centric in life sciences Article5 years ago
-
2021 global life sciences outlook Article4 years ago
-
Radical interoperability Video5 years ago