Intelligent biopharma Forging the links across the value chain
20 minute read
03 October 2019
The pace and scale of medical and scientific innovation is transforming the biopharma industry. The need for better patient engagement and experience is spurring new business models. AI is rising across biopharma.
The rise of artificial intelligence across biopharma
The pace and scale of medical and scientific innovation is transforming the biopharma industry. The need for better patient engagement and experience is spurring new business models. Data generated, captured, analysed and used in real time by innovative medical devices is biopharma’s new currency. A key differentiator for companies is the extent to which they are able to generate insights and evidence from multiple data sources. Consequently, digital transformation is a strategic imperative. This report outlines how artificial intelligence-enabled technologies will impact the biopharma value chain and accelerate biopharma’s digital transformation.
Learn more
Explore the Life sciences collection
Explore the AI & cognitive technologies collection
Download the Deloitte Insights and Dow Jones app
Subscribe to receive related content from Deloitte Insights
Although there is a high level of innovation in the industry, biopharma companies are facing a complex and challenging environment due to increased competition and R&D cycle times, shorter time in market, expiring patents, declining peak sales, pressure around reimbursement and mounting regulatory scrutiny. As we have shown in our series of reports on ‘Measuring the return from pharmaceutical innovation’, these factors are contributing to an alarming decline in the projected return on investment that large biopharma companies might expect to achieve from their late-stage pipelines, threatening their long-term futures.1
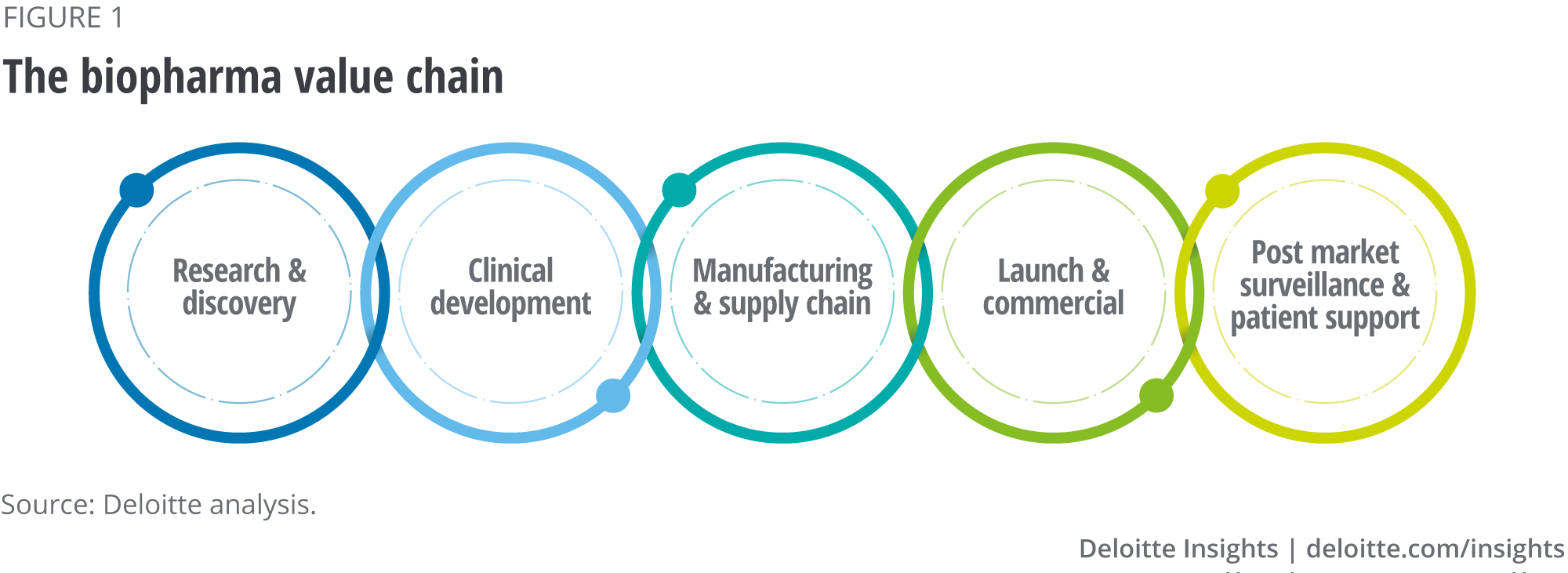
Digital transformation could provide a lifeline to biopharma research and development (R&D) and help reverse this trend. Digital transformation will also impact beyond R&D, as companies look to improve their operational performance, productivity, efficiency and cost-effectiveness across the entire biopharma value chain (see figure 1). Digital transformation will also impact business models, the development of new products and services, and how companies engage with health care professionals, patients and other customers. Ultimately, digital transformation is the next step in the evolution of biopharma companies. Embracing digital transformation will enable biopharma companies to innovate new products and services, engage customers more effectively and execute processes more efficiently.2
This overview report examines the factors that have created a perfect storm for the adoption of AI across the entire biopharma value chain (part 1), the challenges that need to be overcome to optimise the benefits of the industry’s potential AI-enabled transformation (part 2) and summarises the ways that AI is impacting the different parts of the value chain (part 3). We will be examining in more detail the impact of AI on each part of the above value chain in a series of further reports over the coming months.
WHAT IS DIGITAL TRANSFORMATION?
Digital transformation is the use of innovative technologies to reimagine an organisation and drive change management. Digitally mature organisations are committed to transformative strategies that encourage collaboration and new ways of working, are open to taking risks and allow their leaders and employees access to the resources they need to develop digital skills and know-how.
How AI technologies can enable digital transformation
The building blocks of a successful digital transformation include supportive leadership and a culture of collaboration and experimentation. However, the main driving forces behind digital transformation are the emerging technologies that give a company a digital foundation and competitive advantage in data analytics. These include artificial intelligence (AI), blockchain, cloud and virtual reality. The fundamental role of these technologies is to improve the quality of data and information flow and the robustness of insights derived from this data.
Biopharma companies are exploring the capabilities of these digital technologies to further their trans-formation, but many have yet to take full advantage. A June survey by Deloitte and MIT Sloan Management Review found that only 20 per cent of biopharma companies are digitally mature, and that the lack of clear vision, leadership and funding are holding companies back.3 Compared to other industries, biopharma’s digital maturity and adoption of flexible and adaptable leadership and learning models ranked above manufacturing, financial insurance and government, but lagged behind IT, entertainment and telecoms.4
Nevertheless, biopharma’s commitment to digital transformation is increasing. Fifty-eight per cent of survey respondents said that digital transformation is a top management priority, and 79 per cent expect to realise the value of digital initiatives within the next five years.5
WHAT IS AI?
AI refers to any computer programme or system that does something we would normally think of as intelligent in humans. AI technologies extract concepts and relationships from data and learn independently from data patterns, augmenting what humans can do and interacting with humans in a natural way. These technologies include machine learning, deep learning, supervised learning, unsupervised learning, natural language processing (NLP), computer vision, speech and robotics (see figure 2). Advances in wireless technology, miniaturisation and computational power and machine learning architectures are fuelling development of more sophisticated and powerful AI tools (algorithms). Many AI products and services use multiple AI technologies. For example, virtual assistant systems are developed using NLP and speech/voice recognition, machine learning and other data analytics technologies.
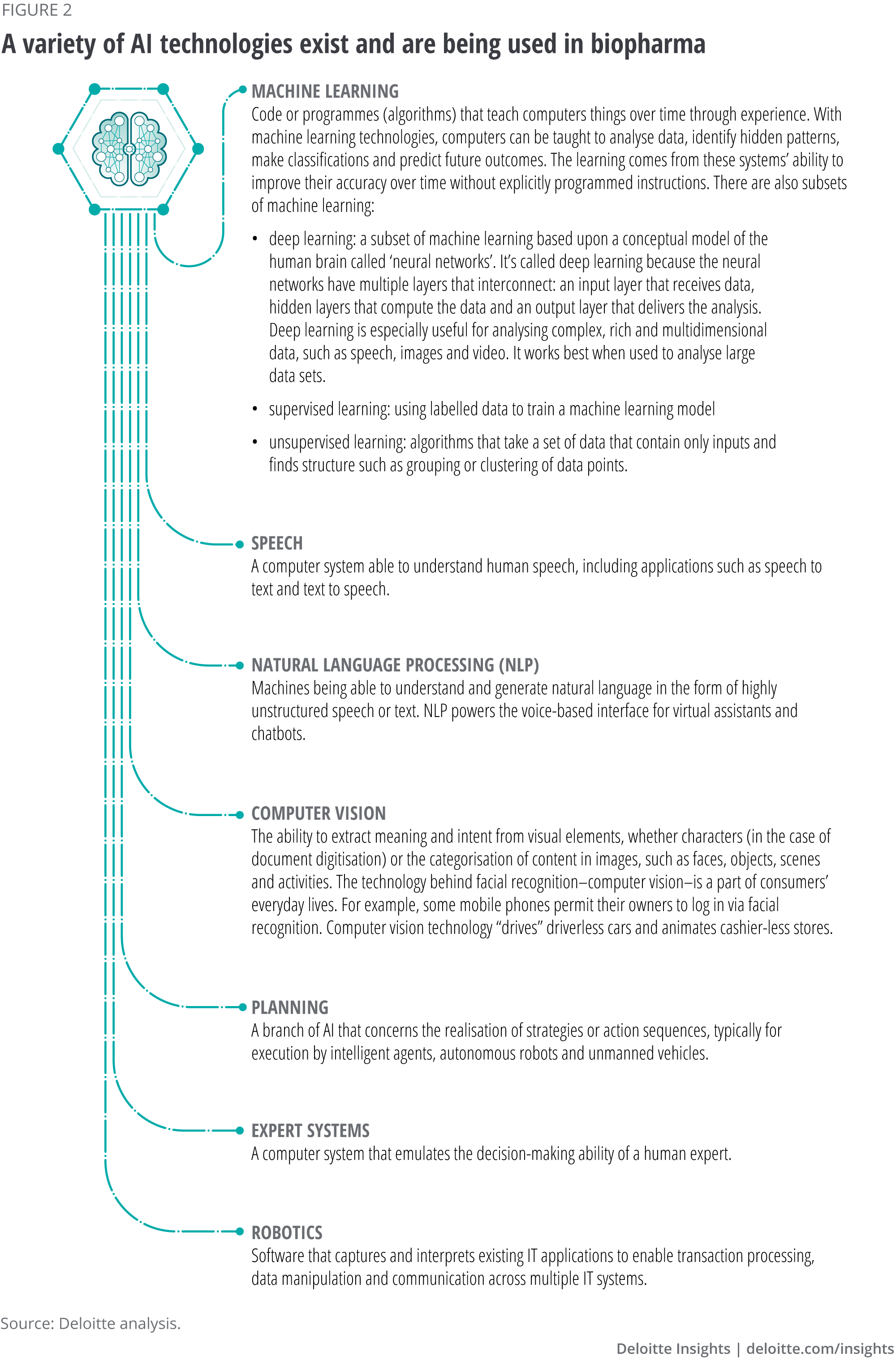
Some biopharma companies are looking to their chief information officers (CIOs) to lead their digital transformation efforts, while others have brought in chief digital officers (CDOs), often from other industries, or chief digital information officers (CDIOs), which combines many of the skills from a CIO and CDO.6 Regardless of the title, digital transformation leaders in biopharma companies need to fully utilise data and emerging digital technologies to transform their business and operating models. The most anticipated and impactful of these technologies fall under the umbrella term of AI.
Currently, machine learning (ML) sits at the core of many AI technologies, with recent advances in ML algorithms and the more complex subset of ML known as deep learning (DL) driving much of the excitement. Until recently, many companies lacked the expertise to maximise the potential of AI technologies, mainly because identifying the right use cases, creating customised solutions and adopting these solutions at scale required access to large data sets, specialised infrastructure and processing power, and a team of data and analytics experts.7 Wide scale adoption also required a high level of investment.
Today, this situation is changing rapidly as early AI adopters such as tech giants and start-ups offer AI-based development tools and applications as products and services, allowing other companies to benefit from AI. Given biopharma’s access to large data sets and the resources to access the required computing power and top technical talent, those companies who are willing to invest early are poised to capitalise on AI.
The market for AI in biopharma
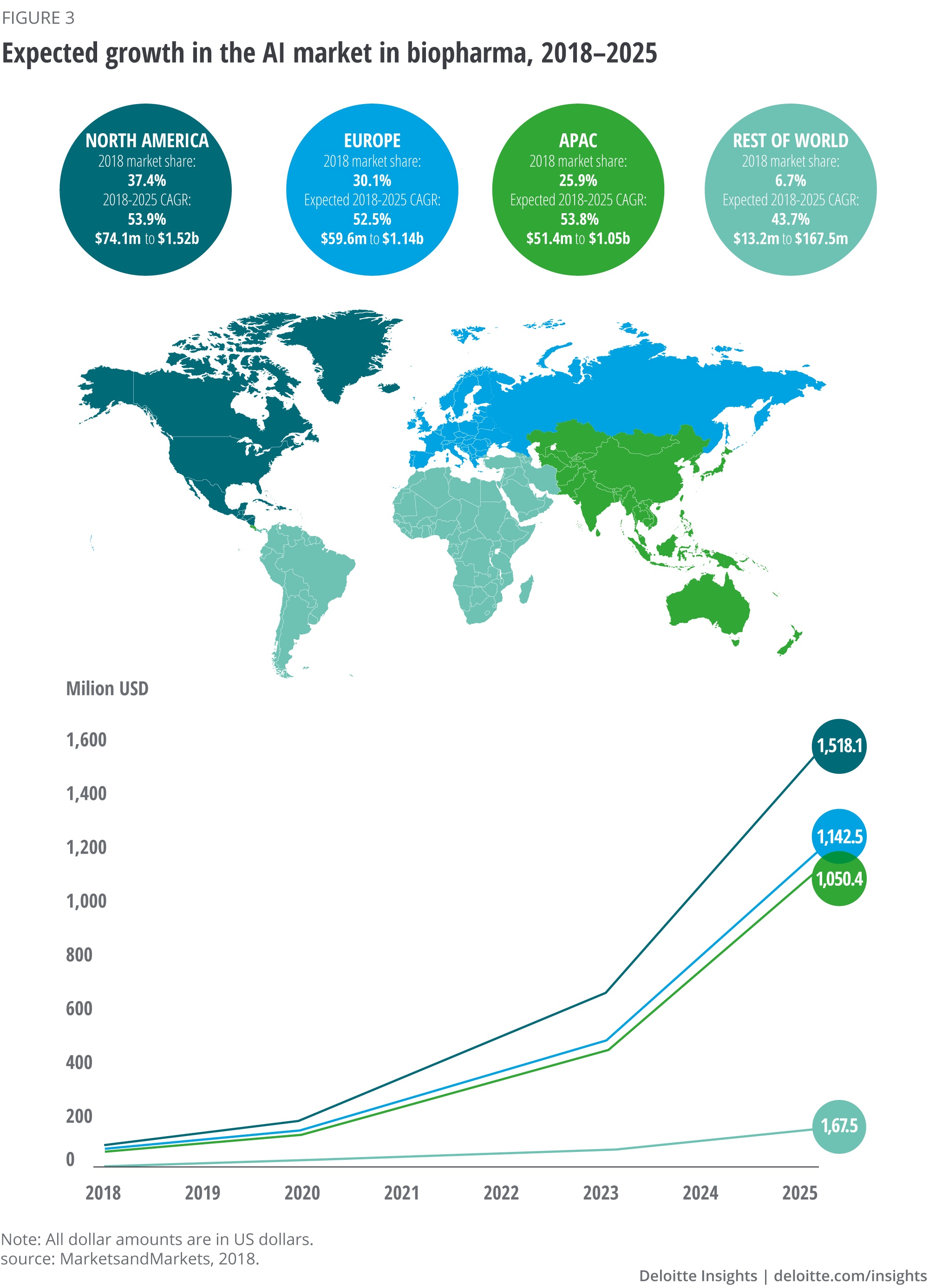
According to MarketsandMarkets, the market for AI in the biopharma industry is expected to increase from US$198.3 million in 2018 to US$3.88 billion in 2025, with a compound annual growth rate (CAGR) of 52.9 per cent.8 These values vary across the four regions: North America, Europe, Asia-Pacific (APAC) and Rest of World (RoW), which includes South America, Africa and the Middle East (see figure 3).
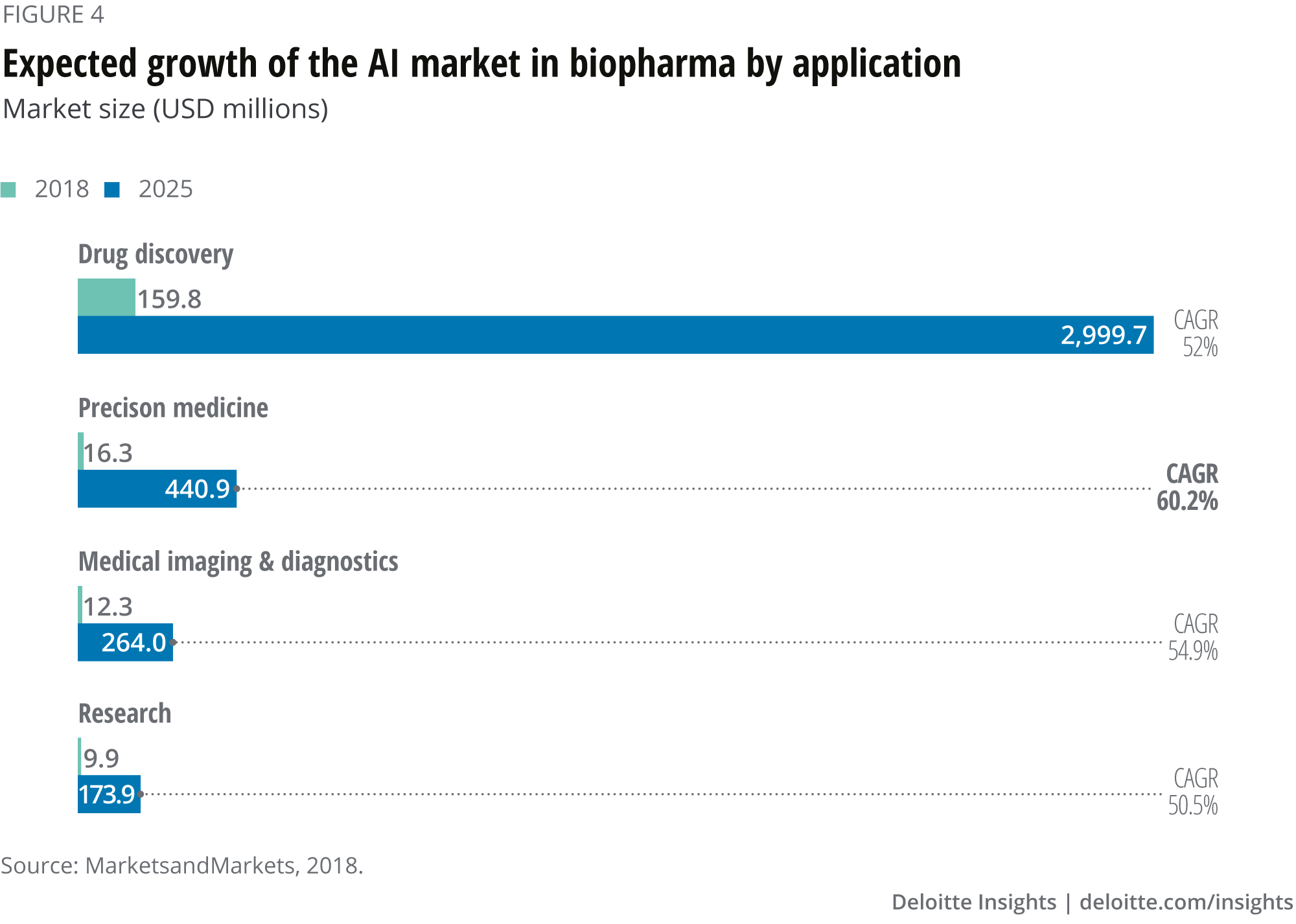
The four applications projected to drive the majority of the AI market in biopharma between 2018 and 2025 include: drug discovery, precision medicine, medical imaging & diagnostics and research. Drug discovery accounted for the largest market size during the forecast period, increasing from US$159.8 million to US$2,999.7 million in 2025, at a CAGR of 52.0 per cent. The market for precision medicine is expected to grow with the highest CAGR over the forecast period of 60.2 per cent, from US$16.3 million in 2018 to US$440.9 million. Medical imaging & diagnostics and research are also expected to grow at CAGRs over 50 per cent (see figure 4).
Factors driving the growth of AI across the value chain
Several factors are contributing to the growth of the global AI market in biopharma and the potential for digital transformation. From a technology and data perspective, three factors in particular are driving progress towards transformation: increasing data volumes, computing power and decreasing costs of computing.
Data generation is increasing in volume exponentially
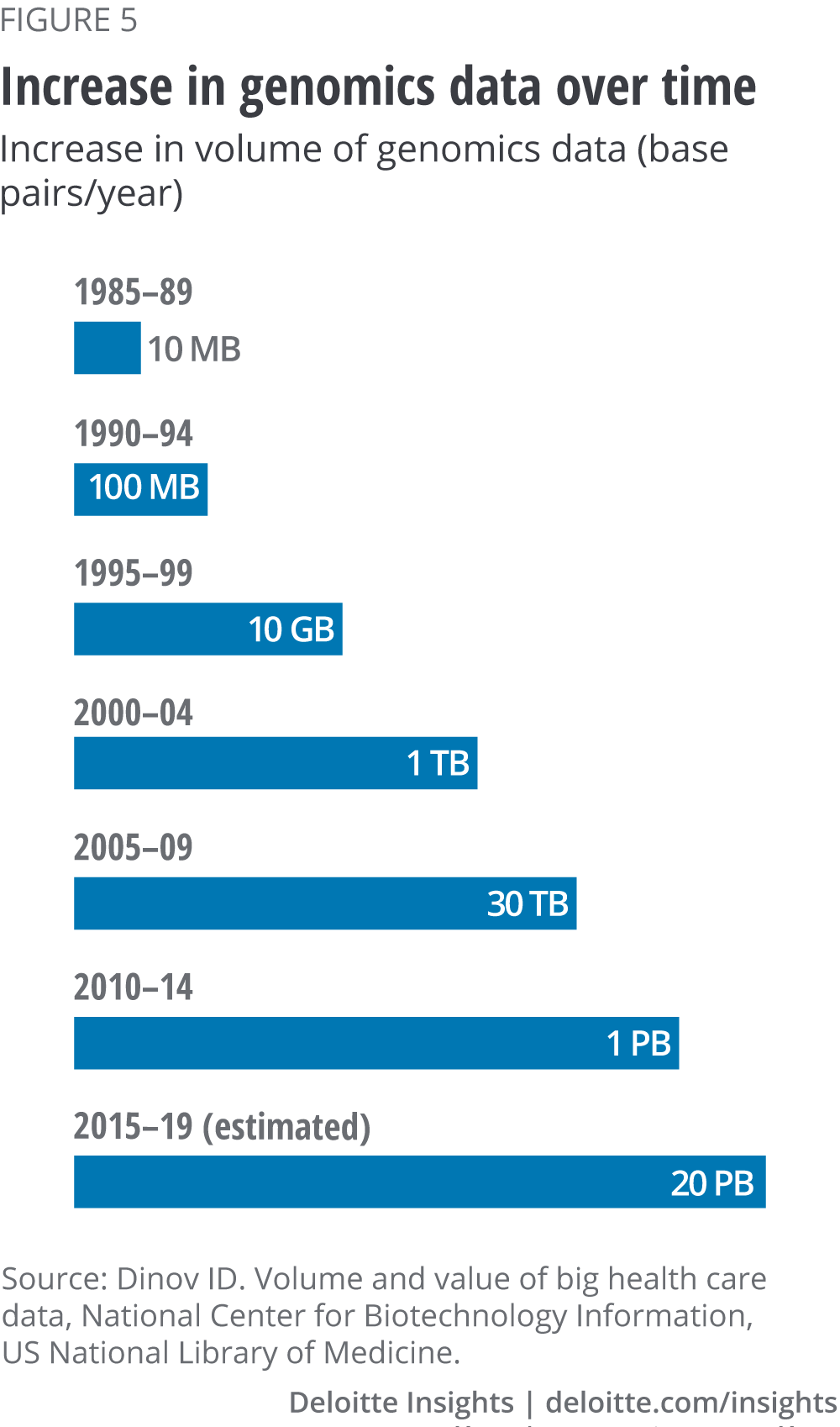
The volume of health care data produced is growing exponentially. For example, the amount of genomics data generated in recent decades has increased from approximately ten megabytes per year in the mid-1980s to over 20 petabytes from 2015–19, an increase of over nine orders of magnitude (see figure 5).
Similarly, biopharma companies are producing increasing amounts of data from numerous sources across the biopharma value chain. Much of this data is in the form of real-world data (RWD), which can come from an ever-expanding variety of sources, including electronic health records, medical imaging, insurance records, wearables and health apps, social media, clinical trials and genomic sequencing. However, RWD often exists in silos and needs to be analysed and processed into real-world evidence (RWE) to generate new insights. AI technologies, particularly machine learning (ML), are increasingly used to analyse RWD.
A Deloitte RWE benchmarking survey conducted between January and April 2018 found that 60 per cent of biopharma companies currently use ML to analyse RWD, but 95 per cent expect to use it in the coming years.9 Moreover, 90 per cent of respondents have either already established or are currently investing in building RWE capabilities for use across the entire product life cycle, though only 45 per cent currently have capabilities mature enough to do so. The survey also found that adopting and applying RWE is no easy feat, 75 per cent of respondents said lack of receptivity by external stakeholders such as payers and providers is a major barrier to the development of RWE capabilities, 70 per cent cite internal stakeholders’ lack of understanding and 65 per cent, lack of access to the necessary external data.10
Computers are more powerful than ever
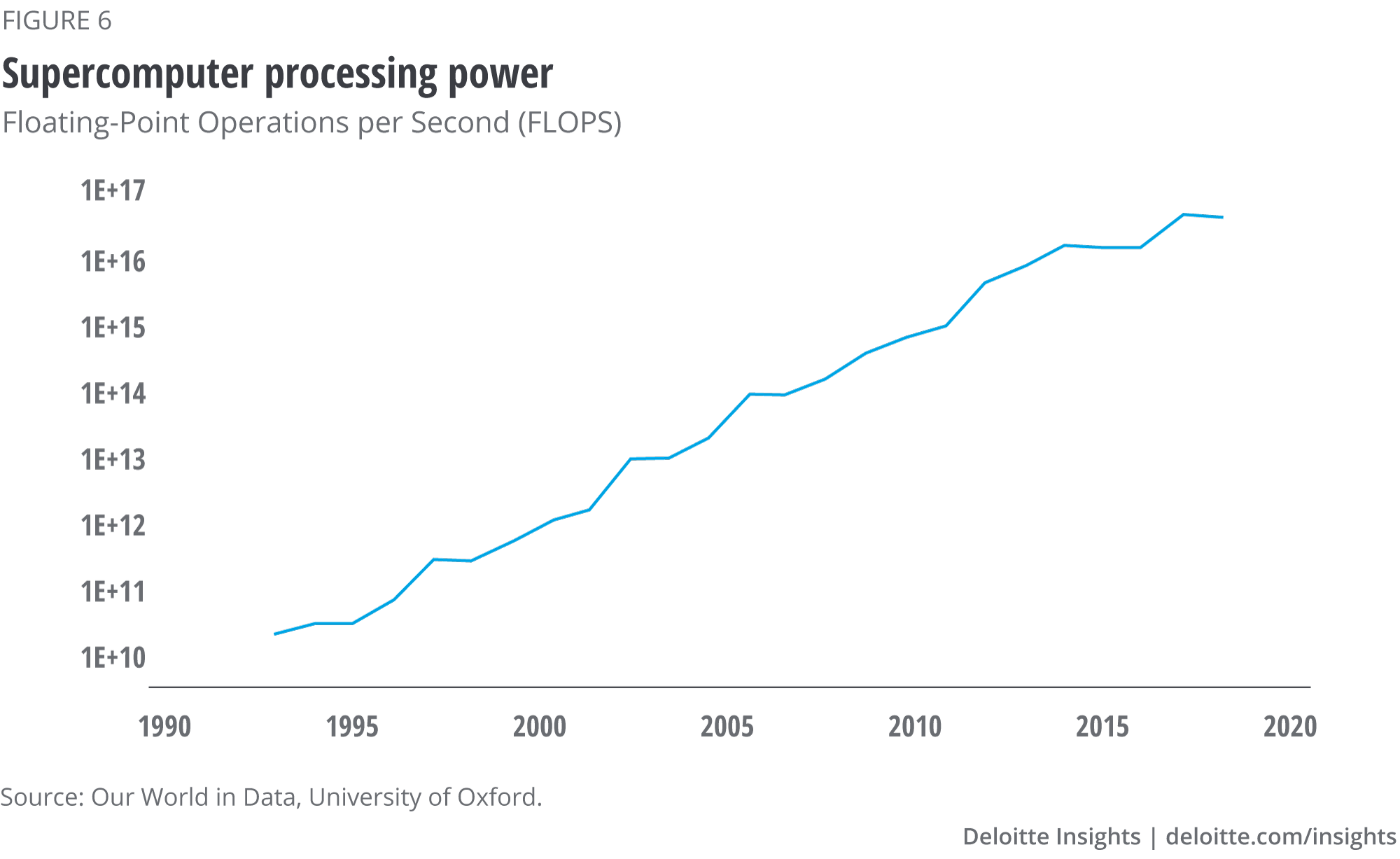
Scrutinising enormous amounts of structured and unstructured data requires algorithms that can analyse sources across the biopharma value chain. It also requires an incredible amount of computing power, which in the past decade has also increased exponentially (see figure 6).11 Microprocessor speeds have increased at a similar rate.12
Computing costs have decreased substantially
At the same time, the cost of computing power has decreased exponentially. For example, the cost to sequence the first whole human genome has decreased from nearly US$100 million in 2001 to just over US$1000 in recent years (see figure 7).13 AI-specific chipsets are also decreasing in price, often very quickly. For example, MarketsandMarkets found that a leading AI chipset decreased significantly in price, falling by close to 50 per cent per unit from 2016 to 2017.14 These developments are leading to the adoption for new applications, driving the growth of the AI chipsets market further.

Biopharma companies are poised to exploit these factors through AI adoption
Separately, these factors can drive incremental increases in efficiency and productivity in bio-pharma. However, incremental increases are not enough for an industry that needs to evolve and transform digitally. Biopharma companies need to act now, as the combination of these factors has created a perfect storm in an industry ripe for transformation. If biopharma is to take full advantage of these factors to transform digitally, there are significant barriers to overcome, which include shifting to a cloud-based computing and storage IT infrastructure. In the next section, we will look at some of these challenges in more detail, focused on themes that run across the biopharma value chain and provide opportunities to drive the adoption of AI technologies.
The challenges and opportunities in AI adoption
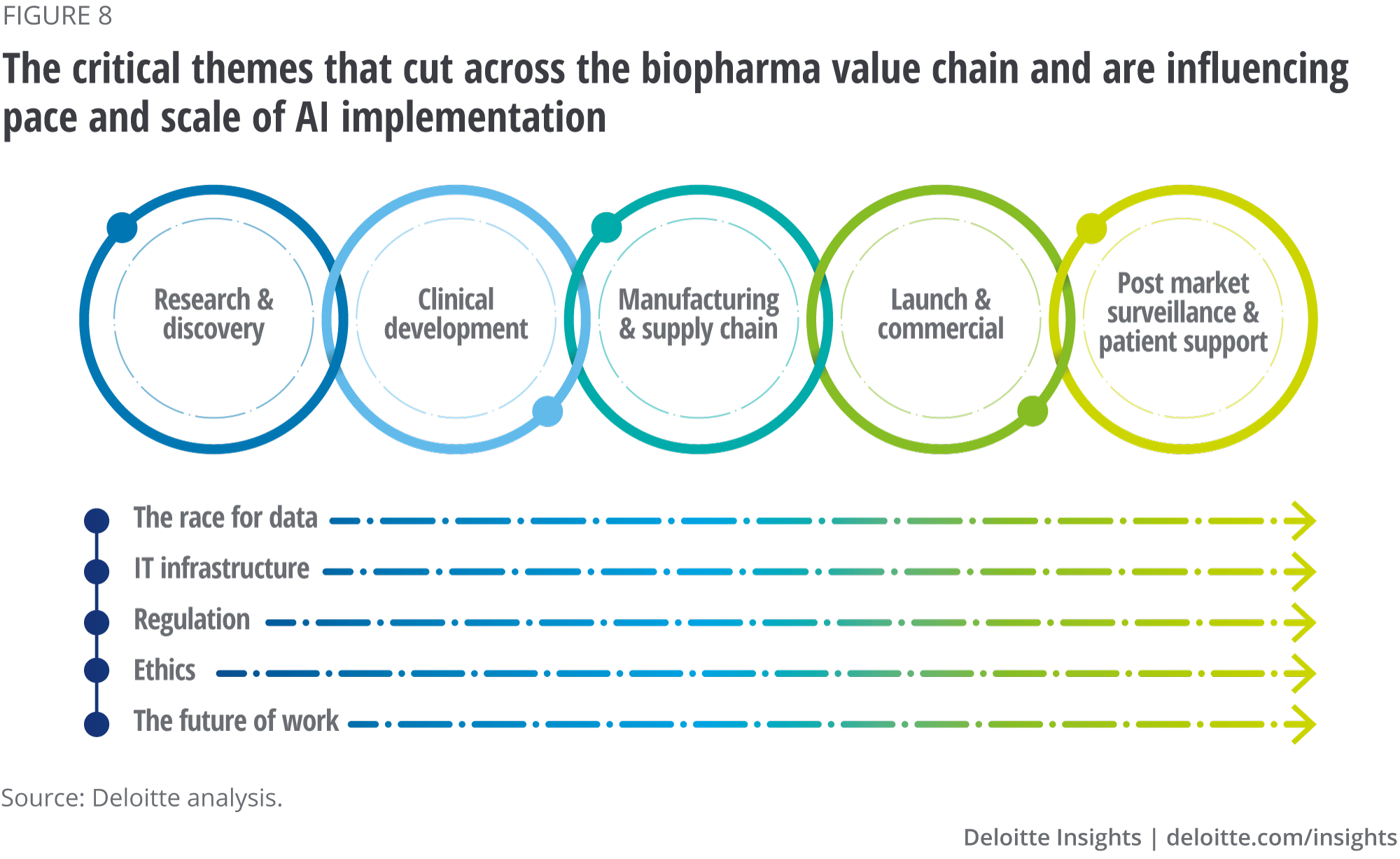
AI technologies will drive digital transformation in biopharma. The rate that biopharma companies improve their use of data, modernise their IT infrastructure, navigate the evolving regulatory landscape, adopt a rules-based approach to data ethics and respond to the impact of AI on the future of work will determine which biopharma companies thrive in a digitally transformed industry. These themes (see figure 8) represent both challenges and opportunities for the industry.
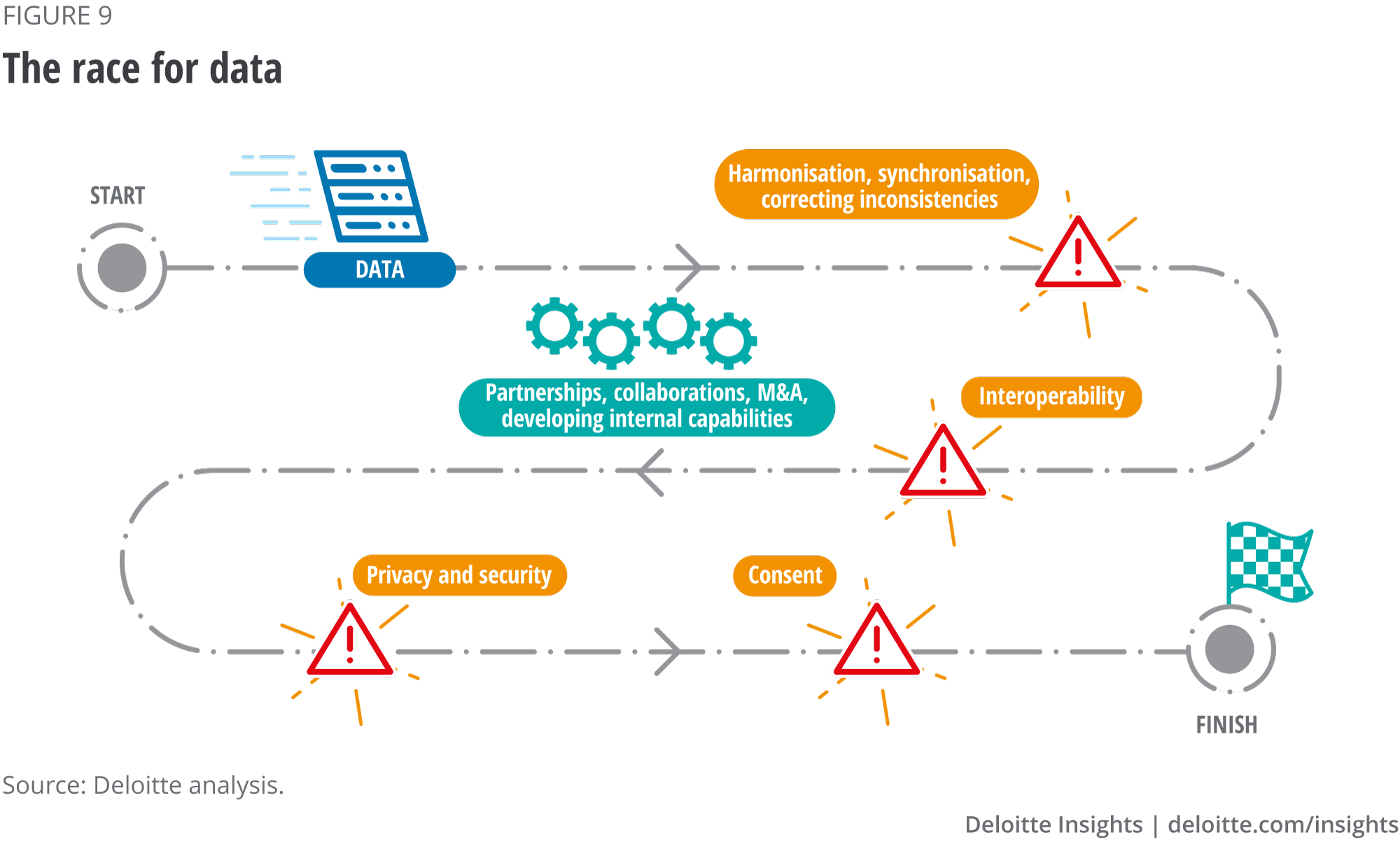
The race for data
If biopharma companies are to capitalise on the availability of AI technologies, they will need to maximise the utility of the huge datasets already at their disposal and identify, access and integrate new sources of RWD. Winning the race for the highest quality data will be the deciding factor in determining which biopharma companies survive and thrive in a digitally transformed world.
The need for robust, reliable, curated data
AI technologies require robust sources of data in order to train AI algorithms effectively and give users confidence in the performance of the technology. The data sources must also be identified, processed and stored in a manner that allows for efficient analysis. However, the large volumes of data created by the biopharma industry are often unstructured, which limits the value that can be extracted. Quality and reliability of data sources vary widely, with data often in the form of text, audio, video and images, which current AI technologies are often unable to process without prior interventions by highly skilled humans. Such interventions include harmonising disparate data sets, synchronising data types and data models, correcting inconsistencies and inputting missing values so that data can be used for analytical purposes.
Data interoperability
Interoperability refers to the extent to which systems and devices can exchange and interpret shared data over wired and wireless networks. It is a significant challenge for all stakeholders across the health care ecosystem, including biopharma companies. Most biopharma companies operate across multiple geographies with multiple diverse IT systems and need access to data from many different types of IT systems used by health care organisations and other partners. Interoperability is extremely complex and relies on being able to establish connectivity and communication between devices and IT systems and between data and workflows to enable secure and transparent data exchange through consensus standards and protocols.15 Developing interoperable systems is essential for improving functions across the entire biopharma value chain, particularly around consented sharing of patient data. Interoperability can also help companies engage more effectively with clinicians and patients, improve the track and trace of products in the supply chain, improve clinical decision support, identify patients and improve medication adherence, among numerous other applications. Interoperability is necessary to improve workflow speed and performance, and break down siloed behaviours that inhibit efficiency.
Data privacy and security
The exponential increase in the amount of data generated and analysed by biopharma companies also increases the importance of maintaining the privacy and security of data. As an information-rich industry, biopharma faces a growing threat from criminals and state-sponsored actors to the data it holds on patients, products and research. Health data is particularly prized as breaches of personal health information (PHI) often remain undetected for long periods and can be used to orchestrate financial and medical fraud, identity theft and hostile intelligence gathering (including support to social engineering and phishing attacks). Without appropriate security, highly motivated adversaries could exploit AI-driven systems through data poisoning attacks to harm the integrity of underlying AI models or use model stealing approaches to harvest intellectual property. These threats necessitate active monitoring of data to preserve their business value. The likelihood and impact of cyberattacks on biopharma’s extensive information assets is moving to the top of the list of business risks confronting senior management.16
Because these types of threats can never be fully eliminated, persistent attention to the privacy and security of data are paramount for biopharma companies. This involves multiple moving parts, including people, governance, organisations, process and controls, technology and intelligence. Another pressing concern is managing the proliferation of laws and regulations in the sphere of data protection and privacy, with almost every government developing or strengthening its own privacy and security legislation.
How to win
With data the new currency in the life sciences industry, biopharma companies are racing to secure access to as much data as possible, either through partnerships, collaborations, mergers and acquisitions (M&A) or developing internal capabilities (see figure 9). However, this can be an uphill battle, at least with regard to patient data. Biopharma companies need to ensure that any patient data they use has specific consent to be used for the specified purpose, and that it is private and secure. Improving information exchange between all stakeholders on threats, incidents, vulnerabilities, best practices and mitigation strategies is an imperative. Biopharma companies also need to commit to cybersecurity education across all tiers of the organisation and supply chain, and ultimately with customers.17
As noted above, interoperability is crucial to the success of biopharma’s digital transformation, and open data and technology standards are key components of this transformation. Tackling the interoperability challenge can be accelerated through the adoption of open platforms, based on open data standards. A key solution to improving the security of data is through using cloud computing power and storage ability (see figure 10). Indeed, cloud services, edge computing, data warehousing and data lakes can all help improve the performance of AI systems.
Updating IT infrastructure
While AI technologies rely on data to learn, improvements in the computer systems and infrastructure that perform the algorithms, including the hardware, software and services, are key factors enabling the rise of AI.
Improvements in hardware, software and support
Computer hardware, including processors, memory and networks, require substantial amounts of computing power to run AI software programmes. As AI software becomes more complex, the required computing power of the hardware increases. Processors need to run more operations in less time, memory boards need to store more information locally and networks need to facilitate the flow of data and information faster.
Software is what allows AI technologies to be deemed ‘intelligent’. Software uses algorithms to receive data from hardware and processes it to form an intelligent response, carrying out the complex operations that simulate reasoning, learning, problem-solving, perception and knowledge representation. Moreover, a key challenge is the ability to generate confidence in the algorithm. In traditional software development projects, the design of the algorithm is transparent and has been subjected to robust verification and validation. However, with ML systems, the system is constantly learning and can often be perceived as a ‘black box’ when testing.
AI technologies require installation, training and support. Deploying and integrating AI hardware and software into a biopharma’s current IT infrastructure can be a complex task that requires highly skilled talent, which many biopharma companies currently lack. Similarly, support and maintenance services that maintain or replace the IT infrastructure to an acceptable standard, can also be expensive and time consuming to keep up to date.
Moving to the cloud to enhance efficiency across the value chain

Traditionally, biopharma companies have stored and analysed their data locally (‘on premises’). However, many biopharma companies are moving away from their traditional IT infrastructures and using cloud computing and data storage for analytics and predictive modelling. Utilising a third-party provider for software as a service (SaaS), infrastructure as a service (IaaS) and platform as a service (PaaS) can have numerous benefits compared to storing and analysing data on premises, including helping to meet data security, privacy and regulatory requirements (see figure 10).18
Managing data in the cloud also eliminates the need to continually update local IT infrastructure and can be cheaper than storing locally.19 This effectively allows small- and mid-sized biopharma companies to reduce their operating expenses and compete with larger companies.
The multiple offices and research facilities of large biopharma companies are often spread across different countries, and this organisational complexity can often impede collaboration. This can be overcome by using cloud computing to enable employees from the same company to collaborate much more effectively. Cloud computing can also help improve collaboration between biopharma companies, smaller biotech companies, research laboratories and academic institutions located anywhere in the world. It also allows companies to process substantial amounts of data faster than on-premises IT infrastructure.20 Deloitte Global predicts that in 2019, all industries will accelerate their use of cloud-based AI software and services. Among companies that adopt AI technology, 70 per cent will obtain AI capabilities through cloud-based enterprise software, and 65 per cent will create AI applications using cloud-based development services.21
Data security is another factor influencing the move to the cloud. Much of the data biopharma companies hold is sensitive, including patient data, or is related to intellectual property. Encryption, cloud data protection gateways, combination passwords, firewalls, cache patterns and other cloud security technologies can protect sensitive information.22 Similarly, utilising a hybrid cloud can be advantageous, which allows biopharma companies to store their core confidential data and applications on a private cloud while selectively utilising the public cloud when appropriate.23
Navigating regulation
The biopharma industry operates in an increasingly complex regulatory landscape. Over the past few years, the industry has seen a proliferation of regulatory changes and a plethora of new regulations still to come into force. At the same time, regulators face the challenge of continuing to protect patients and enhance public health while fostering innovation by responding efficiently and effectively to the exponential pace of change in medicine, science and technology. Regulatory compliance is a cornerstone of product development and commercialisation, giving biopharma companies a framework with which commercial objectives and patient access can be optimised (see figure 11).
Companies, viewing regulation as simply the cost of doing business, risk undermining the potential for innovative medicines achieving regulatory approvals as safely and quickly as possible. Indeed, navigating regulation well and collaborating with regulators can be a differentiator for biopharma companies as both parties embrace AI and other digital technologies to drive the economy, efficiency and effectiveness of regulatory operations.24
Moreover, because personal data is integral to nearly all biopharma business operations, companies need to comply with a variety of data protection requirements including consumer protection and HIPAA legislation as well as international laws such as Europe’s General Data Protection Regulation (GDPR). GDPR is aimed at standardising and strengthening the protection of personal data, including strengthening the rights of individuals to be better informed about how their data are to be used, it also sets out clear responsibilities and obligations on health care professionals and companies using such data, with stringent penalties for infringements.
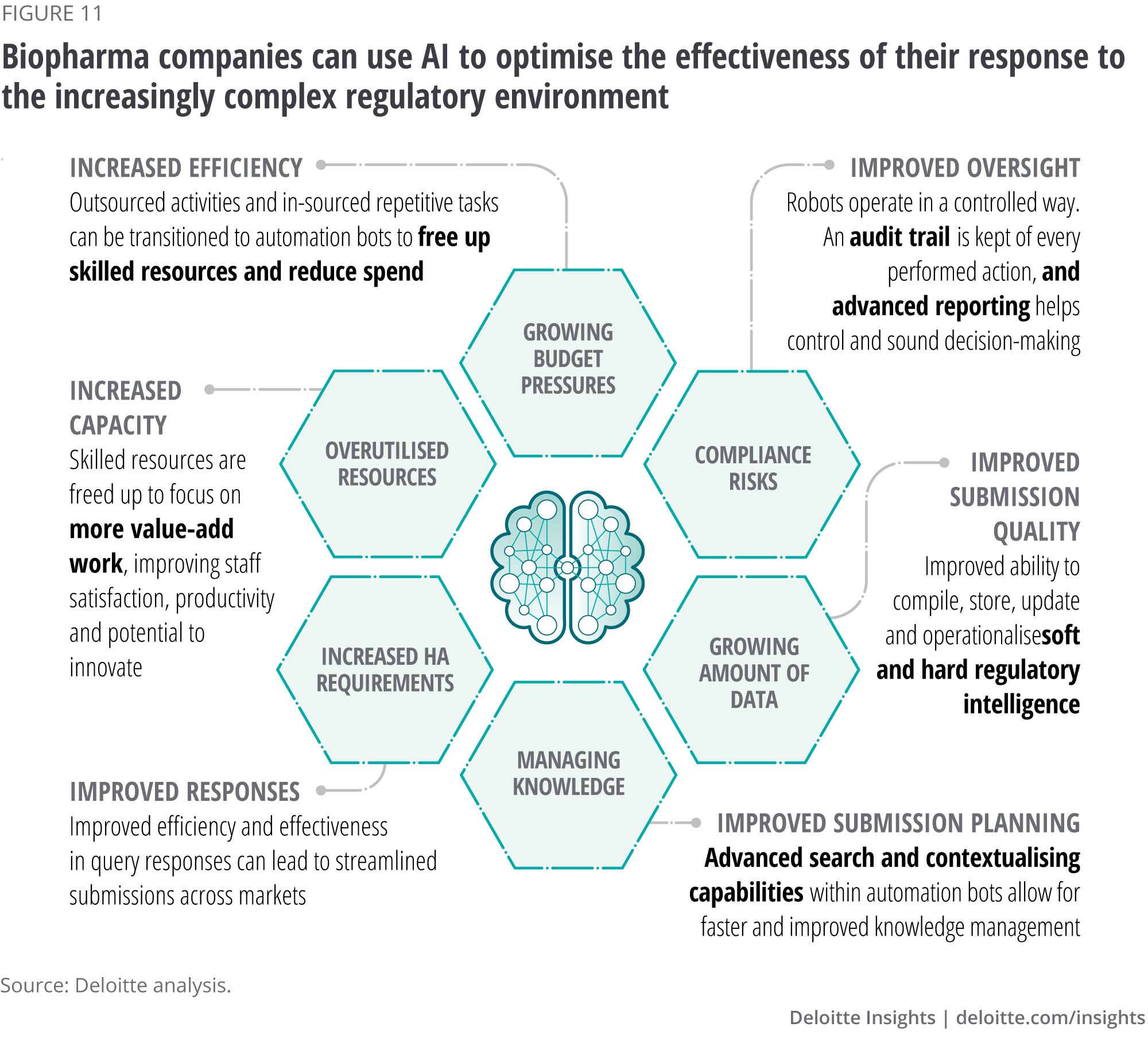
AI and other digital technologies can help regulators increase their efficiency by automating manual tasks, optimising their inspection and enforcement efforts, and accelerating the analysis of large volumes of data. For example, NLP/NLG can be used to analyse and process much of the raw data submitted to regulators. In practice, unstructured data are transformed into structured data, which are then processed (and potentially translated) through an algorithm that generates a narrative. Ultimately, automating numerous processes such as monitoring activities, generating reports, maintaining licenses, managing queries and others that currently consume time and highly skilled resources can reduce costs, improve quality by eliminating human error, improve compliance through better decision-making from better analytics and increase capacity by allowing staff to focus on higher value-add work.
What biopharma companies should consider:
- taking steps to standardise global operations to facilitate regulatory convergence
- increasing the transparency of operations and data to highlight to regulators the benefits of partnerships, as well as improve public trust in the organisation
- transforming culture, processes and operating models to embrace the newfound interconnectivity of the regulation.25
These approaches could lead to the implementation of a robust regulatory intelligence platform within life sciences regulation that leads to a more collaborative and consistent approach to regulation globally. Currently, regulators require manufacturers of AI algorithms to resubmit clearance applications when major modifications are made to software, as regulators are ill-equipped to work with algorithms that constantly change through self-learning.26 However, in April 2019, the Food and Drug Administration (FDA) published an exploratory white paper with a framework that would allow companies to include plans for any anticipated modifications to their algorithms with feedback required by June 2019.27 Similarly, the European Medicines Agency (EMA) has recommended the establishment of an AI test ‘laboratory’ to explore how AI and other digital technologies can be exploited in decision making across key business processes.28 Biopharma companies should therefore think about engaging proactively with the FDA, EMA and other regulators to ensure future guidance from regulators is fit for purpose.
Ethical AI implementation
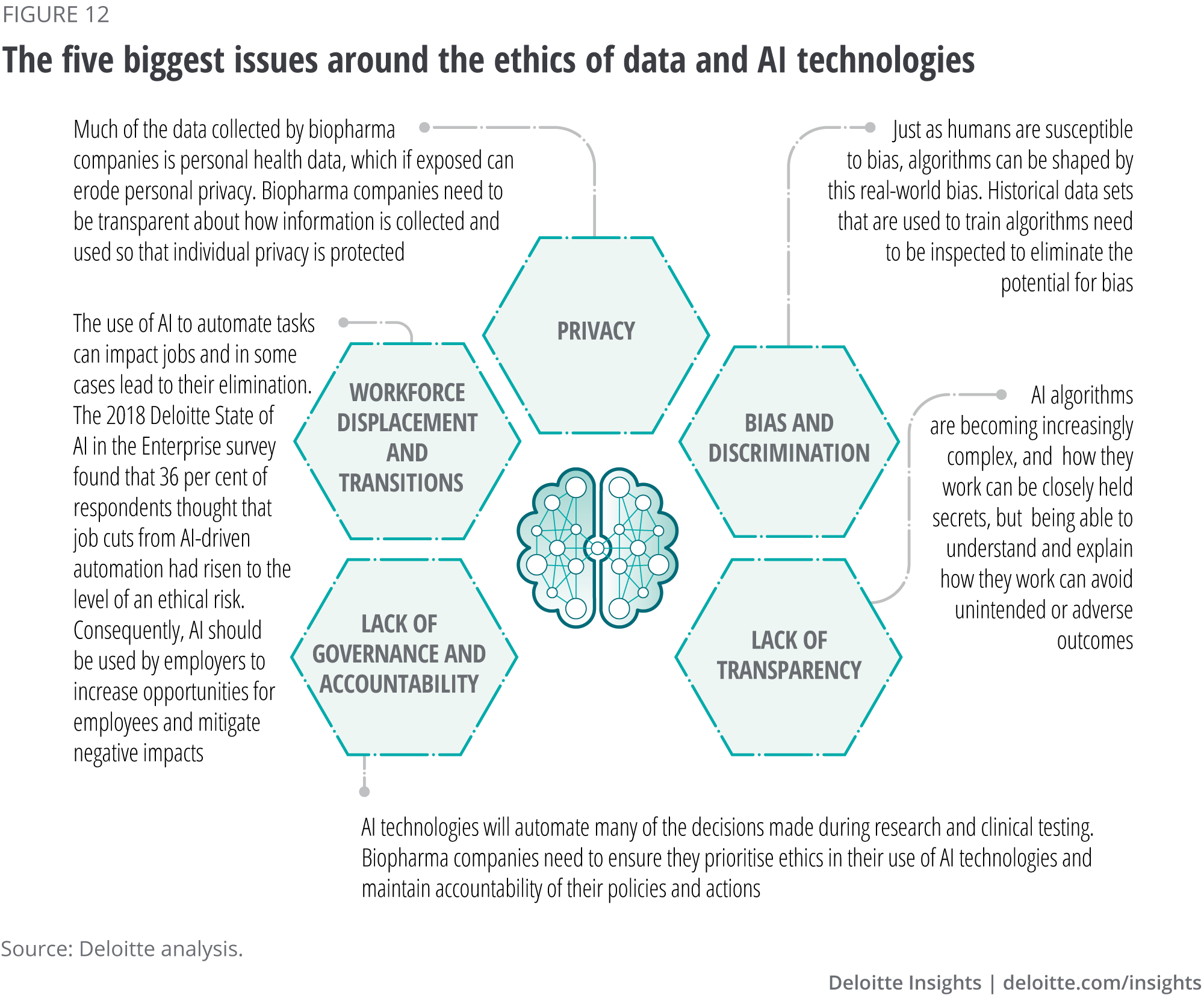
As AI technologies become more powerful, so do the potential for unintended or adverse outcomes. It is every industry’s responsibility to take steps to ensure ethical concerns are addressed. The questions that surround AI ethics can be difficult, and the operational aspects of addressing AI ethics are complex, resulting in five main issues around the ethics of data and AI technologies (see figure 12).29 30
Governments, technology companies, industry, academic institutions and others have started laying the foundation for ethical AI use. For example, technology companies and tech giants are creating AI tools and platforms and have developed ethical guidelines to govern the use of AI internally as well as guide other enterprises. Governments and regulators are also beginning to play a crucial role in establishing policies and guidelines to tackle AI-related ethical issues. For example, the European Union’s GDPR requires organisations to be able to explain decisions made by their algorithms and allow individuals to request their data be anonymised, deleted or forgotten. Other government initiatives include setting up AI ethics councils or task forces, and collaborating with other national governments, industry and other stakeholders. Consequently, biopharma organisations need to build in ethical considerations as they design, build and deploy AI-powered systems, including testing for and remediating systems that unintentionally encode bias and treat users or other affected parties unfairly. Companies can also build trust with stakeholders by being transparent about their use of AI.31
The future of work
Today, traditional thinking and long-standing organisational cultures are holding biopharma companies back. Tomorrow, AI technologies and digital transformation will result in major changes to roles and responsibilities within biopharma companies, requiring companies to rethink the workforce experience, adapt to employing a more diverse workforce and transform their approach to leadership development.32
The next generation of biopharma talent will need to be agile, digitally literate and open to continuous learning as part of their career development. Technology and capabilities will evolve rapidly, and biopharma employees will need to balance the pursuit of new skills with the application of their current skill sets. The race for talent will be run across industries, as biopharma companies compete with other biopharma companies, consumer technology companies and most other industries for data and analytical talent.
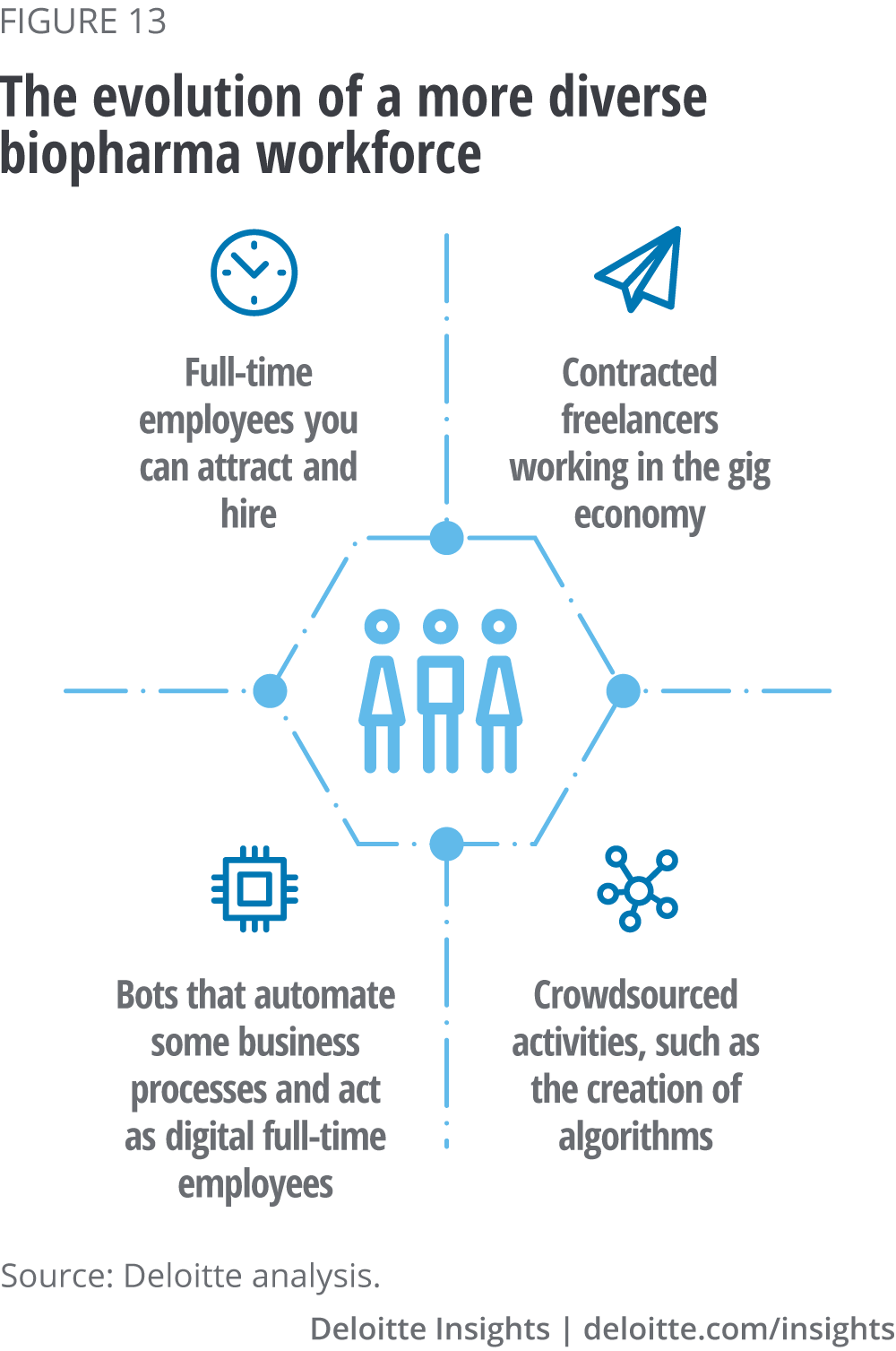
The skills gap is most acute for technical roles such as AI researchers, data scientists and software developers. For example, the August 2018 LinkedIn Workforce Report found that the “demand for data scientists is off the charts”.33 In 2015, there was a national surplus of people with data science skills. Three years later, data science skills shortages are present in almost every large US city. This shortage (151,717 people) is smaller, but growing faster, than the national shortage of software development skills (shortage of 212,838 people). But three years ago, software development skills were already in shortage nationally.34 As only large companies with deep pockets will have the resources to keep industry-leading AI talent on a permanent basis, biopharma leaders will need to embrace a more diverse workforce that has evolved from the traditional employer-employee relationship, in part due to the impact of AI technologies (see figure 13).35
Automation from AI-enabled bots will replace or augment work that humans have traditionally done, allowing employees to focus on higher-value activities while machines take on repetitive tasks that they can do faster, cheaper and more accurately. As evidenced by current trends, bio-pharma companies will initially look to capitalise on AI through partnerships and collaborations with multiple companies, from AI start-ups to large tech. However, to remain sustainable and become an employer of choice, biopharma companies need to create a workplace where “profit meets purpose, talent trumps technology and the social enterprise reigns supreme”.36
A Deloitte 2018 survey of AI early adopters showed that the most popular path to acquiring AI capabilities is also the easiest: enterprise software with integrated AI. Overwhelmingly, this software is cloud-based, either through public or private cloud deployments. Fifty-eight per cent of survey respondents globally were using this approach. Deloitte Global estimates that by 2020, about 87 per cent of AI users will derive part of their AI capabilities from enterprise software with integrated AI. While enterprise software and cloud-based development platforms can provide an effective gateway to AI, they are not a substitute for having at least some in-house technical AI talent. Companies also need to understand the challenges AI can help them solve and how it can help solve them. This requires not only technical talent, but also executives who understand business needs and can ‘speak data science’ to technical experts. These translators can help companies ensure they are not just building models more efficiently, but also efficiently building effective models.37

The future of work is being shaped by two powerful forces, the growing adoption of AI in the workplace and the diversification of the workforce to include both ‘on-balance and off-balance sheet’ talent. These forces of change are affecting three deeply connected dimensions of an organisation: the work itself, who does the work and where the work is done (see figure 14). While the full impact that AI technologies will have on the future of work is not yet a foregone conclusion, biopharma companies need to view advances in these technologies as more than merely avenues to drive more efficiency and cost reduction. AI will create opportunities for biopharma companies to redefine roles, activate and reskill their workforce and generate broad and valuable enterprisewide benefits.38
Biopharma’s AI-fuelled future
Digital transformation will result in biopharma companies using technologies to accelerate innovation, streamline processes and eliminate barriers. AI technologies will be at the forefront of this transformation, helping improve efficiencies, power new products or services, enable new business models and create a biopharma industry vastly different from its current iteration.
AI will impact the entire biopharma value chain
AI’s impact will be felt across the entire biopharma value chain, initially by aggregating and synthesising information from data (see figure 15). Below are examples of the likely applications of AI in the different segments of the value chain.
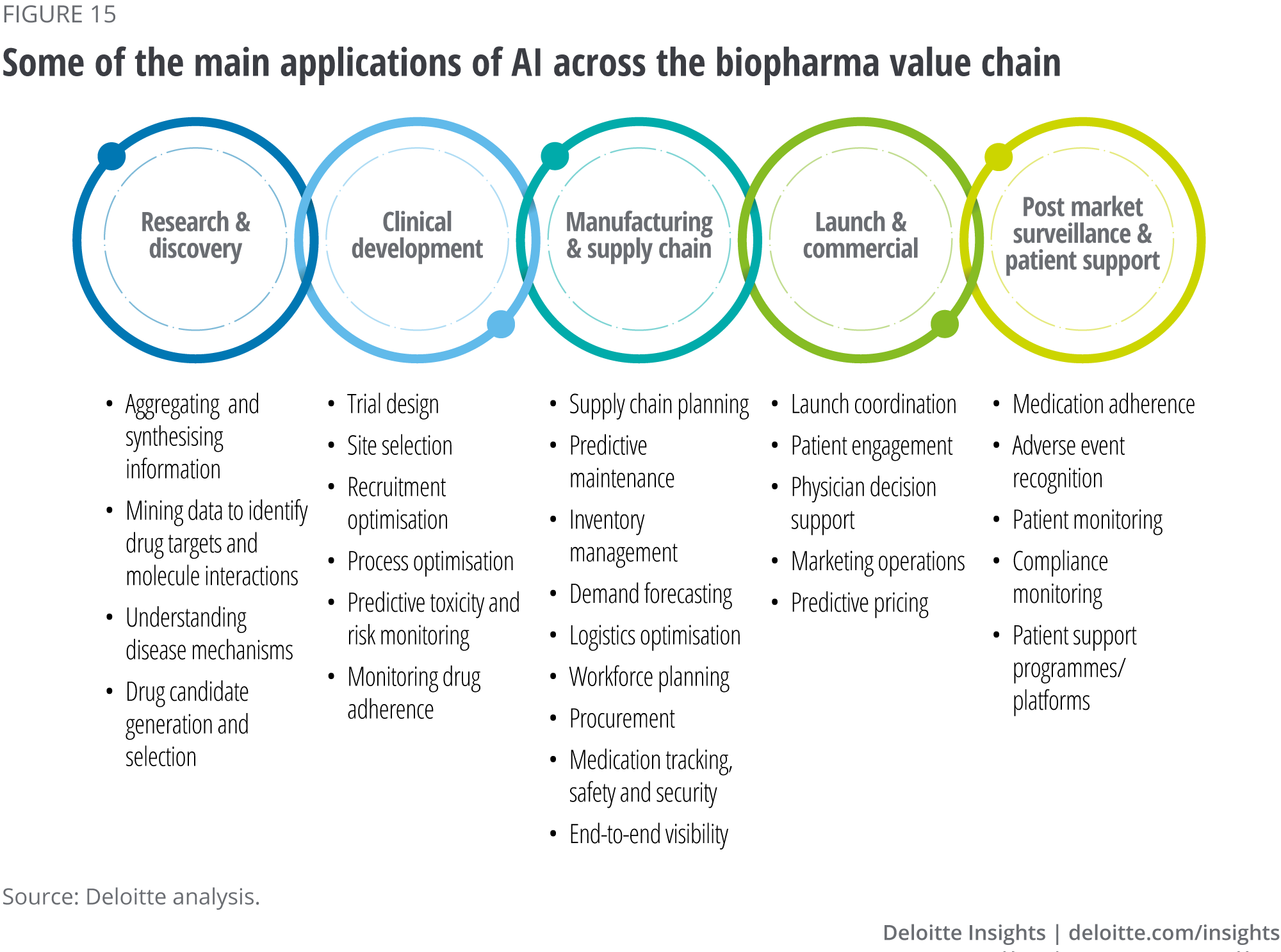
Research and discovery
AI has the potential to drastically improve productivity by applying AI and computational biology to drug discovery and development. This, in turn, will make research and discovery cheaper, faster and more effective. It will reduce the number of failed drugs by identifying patterns in large volumes of data, and it will improve target identification, safety and efficacy assessments by using predictive modelling and in silico testing.
A key role for AI algorithms is in predicting interactions between molecules to understand mechanisms of disease. These mechanisms could in turn help establish new biomarkers; identify, design, validate and optimise novel drug candidates; and identify existing drugs that could be repurposed for other indications. Combining AI with other technologies such as organ-on-a-chip, 3D cell cultures and other cell models that replicate human biology in-vitro, could reduce the need to conduct animal testing and may eventually lead to eliminating Phase 1 clinical trials in healthy volunteers.39
Currently, over 148 start-up companies and over 33 large biopharma companies – either through partnerships or on their own – are applying AI to drug research and discovery.40 41 Companies are also investigating the potential to use AI technologies to design and run preclinical experiments, which could help shorten the overall time it takes to discover, develop and launch a new drug.
Clinical development
Clinical trials are ripe for transformation as they are increasingly slow, expensive and involve a large number of manual processes. In addition, many trials are never completed. In 2018, over 30,000 clinical trials were registered with ClinicalTrials.gov, with nearly 300,000 trials registered since the year 2000. However, fewer than 5,000 trials published results in 2018, and fewer than 35,000 registered studies have published results since 2000.42
Biopharma companies are currently exploring the use of AI technology applications to optimise their clinical trials including improving trial design and patient recruitment, analysis of both trial results and real-world evidence, and automation to increase the speed and efficiency of results publication. Successful implementation could result in reduced development costs, increased adherence and improved outcomes. Furthermore, the availability of rich data sets and AI-driven analytics could also result in the development of highly effective personalised therapies without the need for large-scale clinical trials.43
Manufacturing and supply chain
Biopharma manufacturing and supply chains produce vast amounts of real-time data that for years have been underutilised. However, AI technologies are poised to impact these processes through real-time data processing and decision making that can make supply chains truly data-driven. The potential for end-to-end visibility in manufacturing and supply chains will allow biopharma companies to improve their supply chain planning, inventory management, demand forecasting, logistics optimisation, workforce planning and procurement. The ability to adjust processes and track medications in real time will allow biopharma companies to manage their supply, quality, safety, security and costs, resulting in more intelligent manufacturing and supply chain decision-making.
The industry is likely to shift away from linear supply chains to open, dynamic, and interconnected digital supply networks (DSNs). DSNs leverage a variety of digital technologies such as sensors, robotic process automation (RPA), blockchain, and advanced analytics to enable greater product visibility, traceability and inventory control. Several companies are already seeing benefits from data-driven supply chains with ML applied to production data to build compliance and quality risk models helping to predict failures and compliance issues. Meanwhile RPA and NLP could automate compliance report creation and filing. Manufacturing and shipping next generation products–specifically cell and gene therapies–will likely require a different set of capabilities than traditional molecules. These capabilities should include advanced analytics and workflow automation to seamlessly coordinate patient cell collection, manufacturing capacities, cold chain logistics and delivery of the viable product to the transfusion centre.
Launch and commercial
End-to-end visibility across commercialisation could have significant benefits to biopharma companies. Using AI technologies can help companies coordinate product launches better, improve patient engagement, help with physician decision support and marketing operations, and predict market access and pricing decisions. Early attempts are already underway to use AI for precision engagement by tailoring behavioural nudges to an individual’s personality, motivations, care journeys and engagement challenges. Customised health care practitioner (HCP) engagement could help ensure that the right HCPs get the right content at the right time. AI can help stratify HCPs into various ‘personas’ based on their habits, preferences and receptivity to marketing messages. Evidence generation to establish proof of value will likely become critical to support reimbursement models for new curative therapies and services.44
Post market surveillance and patient support programmes
Post launch, biopharma companies may also need interoperable health data to track and engage with patients remotely and intervene with personalised treatment options at the right time. This requires integration and analysis of patient data in real time to determine if/when an intervention is needed. Applying AI technologies towards safety will be critical, particularly in detecting potential adverse events or adverse events in real time. Insights into patient monitoring beyond safety will also be important, and compliance monitoring is an area many biopharma companies and start-ups are targeting. AI-enabled patient support programmes could transform biopharma’s relationship with customers, improve enrolment, adherence and retention, and deliver a better overall patient outcome.
Stay tuned for more insights
Biopharma’s use of AI across the value chain is still in its infancy, but the time to invest in its future is now. Indeed, AI is increasingly seen as a critical capability of the digitally transformed biopharma company. Maximising the potential of AI technologies will help drive biopharma companies toward full digital transformation, which – if done right – could catapult biopharma companies into a world where they are able to develop more effective drugs, and where these drugs are discovered, clinically tested and approved faster and cheaper than is currently possible.
Over the next few months, we will look at each link of the biopharma value chain in more detail, examining specific case studies and applying insights from interviews with senior leaders across the bio-pharma ecosystem from major biopharma companies to innovative start-ups, including many companies that are poised to disrupt the traditional biopharma landscape.
Explore more on Biopharma
-
Gain the edge in a fast-moving market Article6 years ago
-
Tackling digital transformation in life sciences Article5 years ago
-
Maintaining value in pharmaceutical compliance Article5 years ago
-
Forces of change in health care Article6 years ago
-
Optimizing biopharma market access Article6 years ago