RWE focus is shifting to R&D, early investments begin to pay off How can others catch up?
17 minute read
11 June 2020
 Jeff Morgan United States
Jeff Morgan United States Karla Feghali United States
Karla Feghali United States Sonal Shah United States
Sonal Shah United States- Wendell Miranda India
Companies that have invested in mature RWE capabilities are seeing returns; others are behind. Results from Deloitte’s third RWE benchmarking study reveal how companies still building their capabilities can catch up.
Executive summary
Deloitte has been conducting benchmarking surveys of the pharmaceutical industry since 2017 to track trends in strategic focus, investments, challenges, and opportunities for delivering value with real-world data (RWD) and real-world evidence (RWE). RWD and RWE have remained a C-suite strategic priority for pharmaceutical companies; however, realization of return on investment varies. In this year’s survey, we are seeing for the first time a stratification of results from companies that have invested in more mature capabilities from those that have less mature organizational and technical capabilities.
Learn more
Explore the health care collection
Visit the Health Forward Blog
Learn about Deloitte’s services
Go straight to smart. Get the Deloitte Insights app.
Across both cohorts, this year’s survey shows a clear focus on using RWE to enhance the R&D process. However, those with more mature capabilities are realizing returns in this area disproportionately. More than 40% of this year’s respondents say recent FDA guidance and statements encouraging RWE use in R&D are driving an increased strategic focus. Others pointed to mounting pricing and market access pressures, the rising cost of drug development, or a combination of these factors driving C-suite focus.
Survey results also show:
- Ninety-four percent of survey respondents believe using RWE in R&D will become important or very important to their organizations by 2022.
- Survey respondents expect a shift in the application of RWE in the next two to three years with the highest impact being in R&D, including supporting regulatory filings and augmenting clinical trials, in contrast to the more traditional applications where they realize value today.
- Most respondents expect a wide range of benefits from using RWE in R&D. So far, the biggest gains have come in the form of cost reduction in post-marketing commitments and executing clinical trials.
- Seventy percent say that a lack of research-grade data is hindering RWE efforts in R&D. This emphasizes the importance of establishing strategic partnerships: More than 80% of surveyed companies are entering into strategic partnerships to access new sources of RWD.
- Almost all companies expect to increase investments in talent, technology, and external partnerships to strengthen their RWE capabilities.
“The future is already here—it is just not evenly distributed”1 is an apropos quote to describe RWD and RWE capabilities across pharmaceutical companies in 2020. Some survey respondents are further ahead in terms of investing and realizing value from RWE capabilities as compared to their peers. Mature companies—that we define as those organizations reporting to have mature capabilities in place already—are more likely to have:
- C-suite executives who already view RWE as a very important strategic priority
- Realized the impact of RWE in newer application areas such as supporting regulatory submissions, building synthetic control arms, and informing the design of value-based contracts
- Reduced clinical trial costs and trial failure rates through the use of RWE in R&D
- Built more robust centralized cloud-based platforms with comprehensive capabilities, including knowledge management and self-service analytics applications
- Entered into strategic partnerships to access new sources of RWD (in fact, all have taken this step)
While some companies are further ahead than others, the industry has yet to realize the full potential of RWE in transforming how medicines are developed, commercialized, and reimbursed. Mature companies have demonstrated that a strong foundation with centralized technology platforms and governance is a critical first step. Companies should also define a clear enterprisewide strategy for their RWE investments inclusive of innovation pilots, a framework to measure ROI, challenge to conventional thinking, and engagement with the health care ecosystem in new ways to elevate RWE’s impact.
Regulators are encouraging the use of RWE
Since our last RWE benchmarking survey, we have seen numerous statements from senior FDA officials as well as draft guidance issued by the FDA on how RWD and RWE can be incorporated into regulatory decision-making.2 The FDA has been actively assessing RWE for regulatory purposes3 and in 2019, released a draft guidance on use of RWE in regulatory submissions.4 The European Medicines Agency (EMA) released the EMA regulatory science to 2025 reflection paper which included promoting the use of high-quality RWD in decision-making as one of its strategic goals.5 Organizations such as the Bipartisan Policy Center published recommendations to advance the generation and use of RWE for regulatory evaluation.6 These are just a few examples of how momentum is continuing to build across the health care ecosystem supporting the expanded use of RWD and RWE in a broader context.
In our first two RWE benchmarking surveys, we saw the industry being optimistic about the increased impact RWD and RWE would have across the pharmaceutical enterprise with a subsegment of the market making bold investments in new technology platforms, partnerships, and organizational capabilities as a result. For our third RWE benchmarking study, we set out to understand if life sciences companies have realized these results and are continuing to make bold moves to leverage RWD and RWE across the enterprise and what the industry’s vision for the future of RWE is. (For details on the survey methodology, see sidebar “Research methodology.”)
Research methodology
Between January and February 2020, the Deloitte Center for Health Solutions surveyed multiple leaders from 17 pharmaceutical companies on their organizations’ RWE capabilities. Survey questions revolved around current and future applications for RWE, areas of investment, strategic partnerships, and use of RWD and RWE in R&D.
More than 40% of survey respondents attribute the heightened sense of importance of RWE within their organizations to recent FDA guidance and statements encouraging life sciences companies to expand the use of RWE in R&D. Dr Janet Woodcock, director of the US FDA Center for Drug Evaluation and Research, has recognized the need to use RWE to improve trials, stating “FDA will work with its stakeholders to understand how RWE can best be used to increase the efficiency of clinical research and answer questions that may not have been answered in the trials that led to the drug approval, for example, how a drug works in populations that weren't studied prior to approval.”7 The greater focus on R&D comes at an opportune time as we have seen the return on pharmaceutical R&D continue to decline to record lows, and the cost of drug development has exceeded US$2 billion.8
This is not to say that market access pressures have gone away. Close to 25% of respondents said that mounting pricing pressures and the need to continuously demonstrate the value of products is driving increased focus on RWE within their organizations. Another 25% of respondents believe that a confluence of factors is driving the heightened importance of RWE.
Companies expect RWE’s importance to grow; some already view it as critical
Consistent with our last survey,9 C-suite leaders in pharmaceutical companies increasingly view RWE as strategically important.
Most survey respondents (70%) believe RWE will become very important to their C-suite by 2022. Interestingly, companies that reported that they have a mature RWE capability in place (almost half of survey participants) said RWE had already reached the highest level of importance to their C-suite today.
This year’s survey results also reflect another consistent trend in the top areas where companies are realizing value from applying RWE. Areas of highest impact today are focused on use cases where there is a long history of leveraging RWE, such as understanding of burden of disease, monitoring patient safety, and comparative effectiveness research. These RWE applications go back decades in some instances, with well-defined methods and data sources for the research, and have played a critical role in the evidence story for products. Most survey participants believe that over the next two to three years, they will see a greater impact with newer applications of RWE, including supporting regulatory submissions, building synthetic control arms, and informing the design of value-based contracts (figure 1). These use cases are more nuanced—in some cases, there are no well-defined approaches and the data required could be different than the data historically used for RWE research.

Across the entire cohort of companies surveyed, these results look similar to those from our 2018 survey. However, a deeper analysis suggests that progress around the use of RWE varies within the industry, especially between companies that have mature RWE capabilities and those that do not. Some mature companies are already seeing the impact of RWD and RWE in newer application areas such as R&D. Companies still building their RWE capabilities are aspiring to see the impact in the next two or three years.
Opportunities exist in R&D, but value is not widely realized yet
Our 2018 survey signaled that the industry was going to increasingly focus on using RWE in R&D. This year, we wanted to assess the level of its importance, biggest areas of opportunity, early successes, and challenges to seeing impact. Survey respondents expect the importance and use of RWE in R&D to increase significantly in the next two years. Ninety-four percent of survey respondents believe using RWE in R&D will become important or very important to their organizations by 2022 in R&D (figure 2).
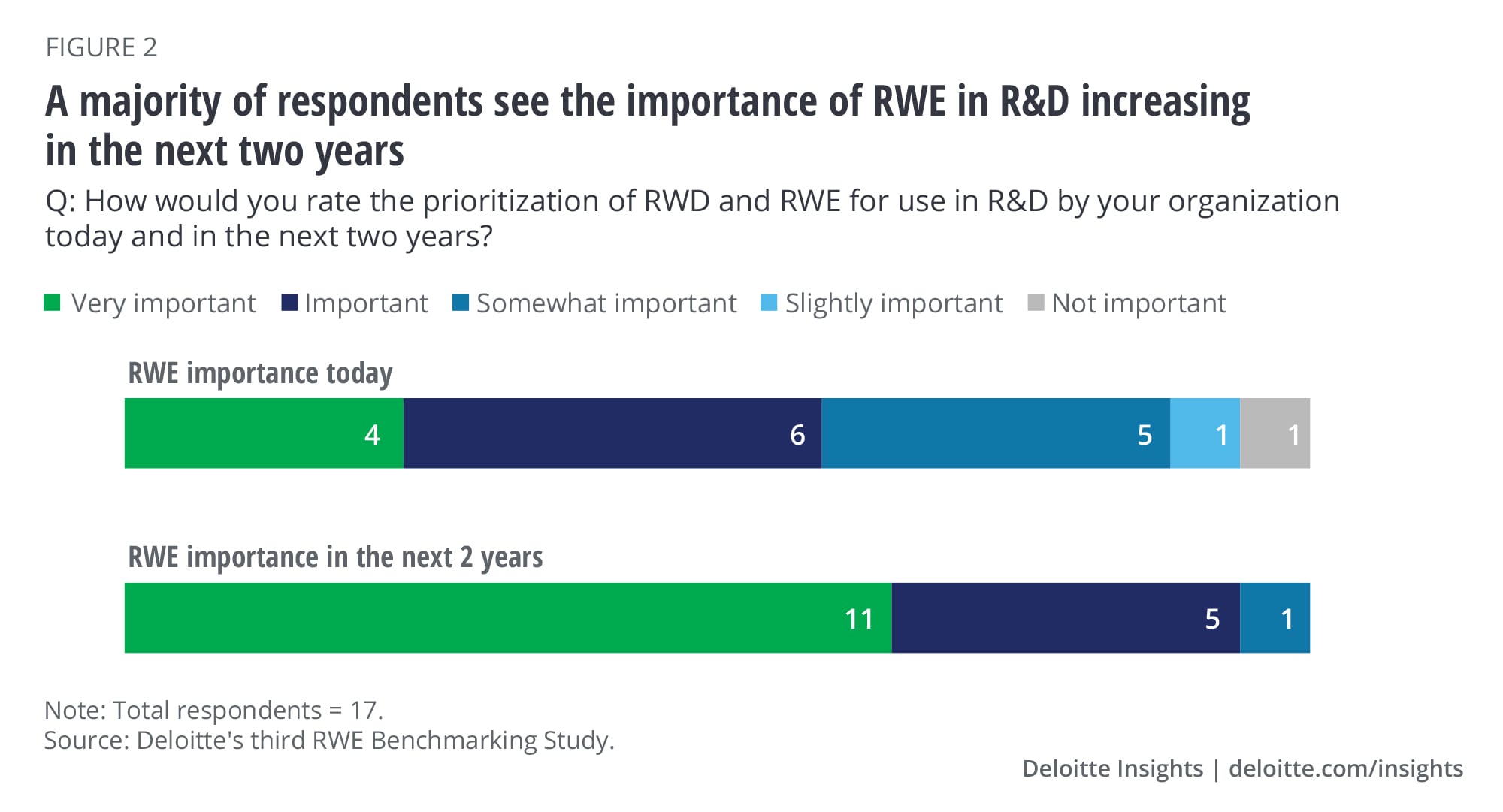
A lot has been written about the significant opportunity that applying RWD and RWE to R&D presents. Using RWD can inform better protocol design, thereby reducing the number of costly protocol amendments, and can enable the creation of synthetic control arms to accelerate trial execution and decrease overall cost. Similarly, RWE can accelerate label expansion and decrease the overall cost of the evidence needed for filing.
Survey data shows most organizations expect a wide range of benefits from applying RWD and RWE in R&D (figure 3), including:
- Better decision-making on target product profiles or development strategy early on (e.g., simulate trials with RWD, understand unmet medical need)
- Reduced cost of supporting post-marketing regulatory commitments
- Faster delivery of drugs to market facilitated by new trial designs (e.g., adaptive trial design, use of synthetic control arms)
- Label expansion of marketed products
- Reduced trial failure rates through improved protocol design and site selection strategy. Deloitte’s research on digital in clinical development suggests companies are already leveraging technology platforms to mine patient records to identify and recruit patients for trials.10
The COVID-19 pandemic has forced several pharmaceutical companies to either delay new studies or halt enrollment for ongoing trials. A survey showed close to one-third of clinical trial sites in the United States expect COVID-19 to impact their ability to recruit patients for new trials or keep patients already enrolled in existing studies.11
Using RWD to understand the current impact of COVID-19 on patient care could allow clinical operations teams to quickly create alternative strategies to mitigate the impact of the pandemic on planned or ongoing studies. In some scenarios, pharmaceutical companies are looking to virtualize studies so they can continue with minimal impact. Using RWD to inform which trials should be considered for virtualization can help accelerate decision-making. Organizations that have access to the right data and the platforms to analyze it quickly are likely best positioned to realize the benefit of RWD in this context.
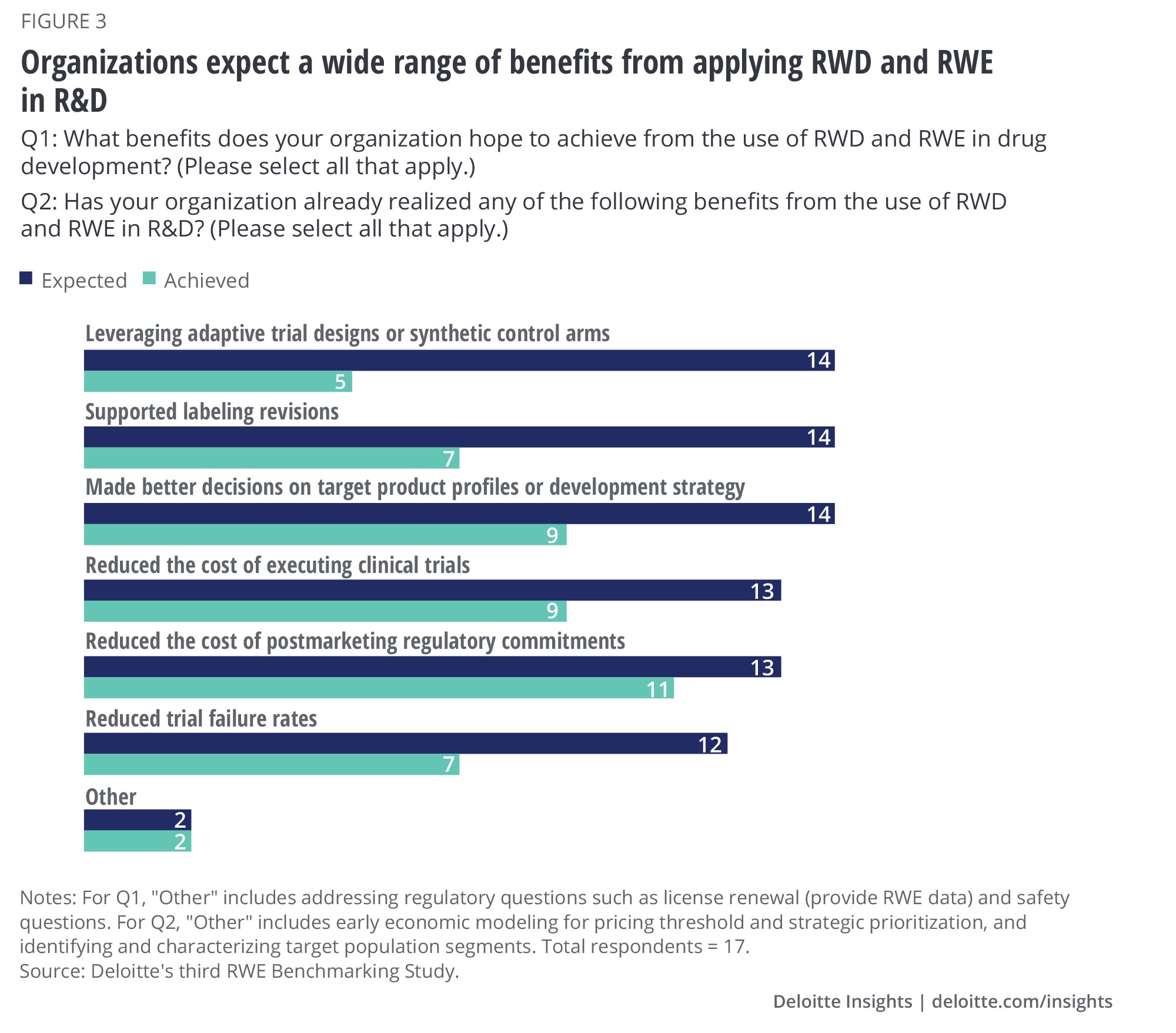
While there is strong consensus that there are significant opportunities for RWD and RWE use in R&D, survey data suggests that many organizations are yet to make the most of its potential in this area. A little more than half of the companies we surveyed reported having already reduced the costs of post-marketing commitments and are making more informed decisions on target product portfolios using RWD and RWE (figure 3). Mature companies are more likely to have also reduced the cost of executing trials and used RWE to reduce trial failure rates. However, the biggest gap between expected benefit and realized benefit was with the use of RWD in adaptive trial designs and synthetic control arms. Both of these areas hold great potential to improve R&D productivity but less than half of the organizations have been able to leverage them to the fullest. Most that have achieved these goals are mature companies. The impact of using RWE for these applications can be tremendous (see sidebar, “RWE to streamline drug development and improve productivity”).
RWE to streamline drug development and improve productivity
Our research points to some examples of how RWE can be used to achieve significant impact in R&D.
Synthetic control arms: Merck KGaA and Pfizer used RWE generated from a retrospective observational study to support results from a single arm trial to demonstrate safety and efficacy of using Avelumab to treat Merkel cell carcinoma. This brought down the approval time to three years, approximately 1.8 years shorter than the median for other drugs in an expedited approval program.12
Expanding label indications: Originally approved to treat female breast cancer in 2015, physicians began off-label prescribing of Pfizer’s Ibrance to male breast cancer patients. Pfizer worked with Flatiron and IQVIA to collectively evaluate EMR and claims data to prove the safety and efficacy of using Ibrance to treat male breast cancer patients. This analysis led to Pfizer receiving US FDA approval in less than a year and at a fraction of the cost of a clinical trial.13
Reducing trial failure rates: Since 2018, Novartis has used TriNetX, a global health research network to analyze RWD obtained from contract research organizations and health care organizations to conduct early feasibility analyses on more than 150 clinical trial protocols. These feasibility analyses are likely to lead to increased probabilities of trial success and shorter recruitment times.14
There are many examples of using RWE in the R&D setting, but respondents indicate that reaching a state where it can be broadly applied will require overcoming several barriers. Seventy percent of survey respondents believe access to research-grade RWD is a major challenge to the success of their organization’s RWE efforts. Concerns around the reliability and accuracy of RWD collection and gaps in real-world data sets are limiting the utility of RWE in R&D. Overcoming this barrier may require looking beyond traditional data vendors and establishing strategic partnerships with emerging vendors, health care providers, other systems of care, or patients themselves to curate high-quality RWD.
The growth of strategic partnerships as part of organizations’ data strategy
More than 80% of surveyed organizations reported entering into strategic partnerships to access new sources of RWD, including all mature companies. At present companies are using strategic partnerships to gain access to high-quality research-grade RWD, particularly for oncology (see sidebar, “Partnering for research-grade RWD”).
Partnering for research-grade RWD
Almost all survey respondents (96%) expect RWD and RWE to have a greater impact on R&D in oncology as compared to other therapy areas. This is likely due the availability of research-grade RWD in oncology and an increasing number of partnerships aimed at harnessing this data.
Oncology centers are collaborating to build real-world data sets to support oncology research. The collaboratives, such as ORIEN, have built de-identified, longitudinal databases that link clinical genomics data (including tumor sequencing data) to clinical data at a patient level.15 Mining such data sets can help build synthetic control arms, expedite patient matching based on biomarker data, and eventually generate regulatory-grade RWE to support approval.
Looking beyond oncology, companies should explore novel approaches to curate or access research-grade RWD. This may require partnering with technology companies, health care organizations, and other stakeholders to access, collect, and build research-grade real world data sets.
During the COVID-19 pandemic, large-scale studies using RWD curated in partnership with hospitals treating patients could accelerate analyses of populations and treatments. In fact, the Observational Health Data Sciences and Informatics (or OHDSI) program, a multistakeholder, interdisciplinary, and international collaborative, held a “study-a-thon” in March 2020 to inform global decision-making about the pandemic. The event resulted in several manuscripts, and the organization has committed to leveraging its data and capabilities to study which ongoing COVID-19 treatments are working.16
Strategic partnerships are also being used to expand access to RWD globally. Our survey results show respondents ranked access to RWD in China, Germany, and Japan as being important to their future RWE strategies (beyond the United States). In China and Japan, regulators are attempting to expand the use of RWE in their review practices.17 Increasing the access to RWD from outside the United States holds great promise but more work needs to be done. The quality of RWD outside the United States varies, and in many cases, it requires significant effort to curate the data to meet the requirements of the use case.
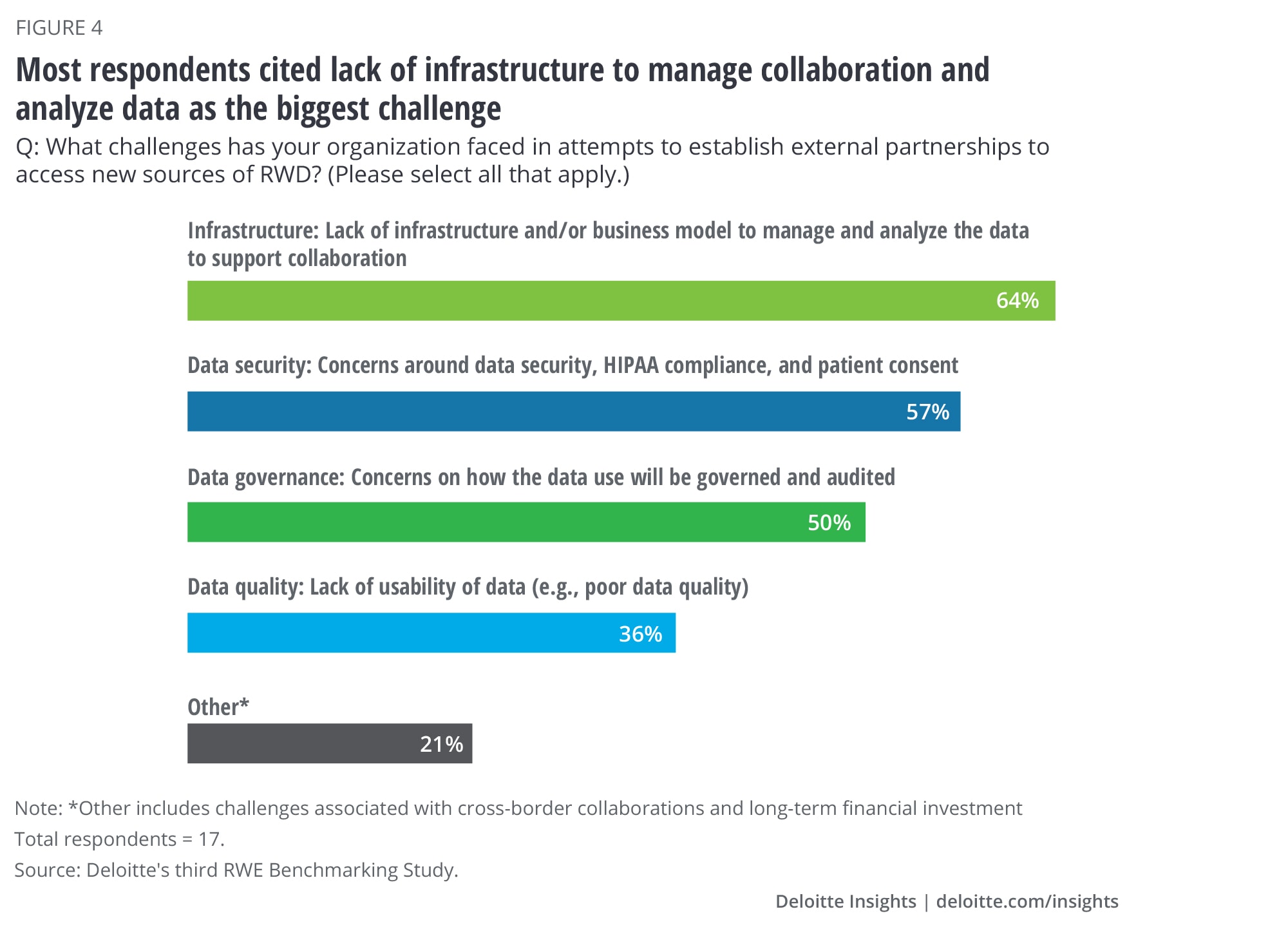
The road to successfully partnering with other organizations isn’t without bumps. Of the 80% of respondents entering strategic partnerships, 64% say lack of infrastructure and a business model to manage the collaboration and analyze data is the top challenge to successful partnership (figure 4). This underscores the need for a foundational technology platform that not only meets the needs of internal stakeholders but also of external partners. Technology alone will not overcome this challenge: Having a dedicated team to manage these strategic partnerships can be instrumental in ensuring the collaboration is established and governed in a way that is mutually beneficial to both parties and value can be realized.
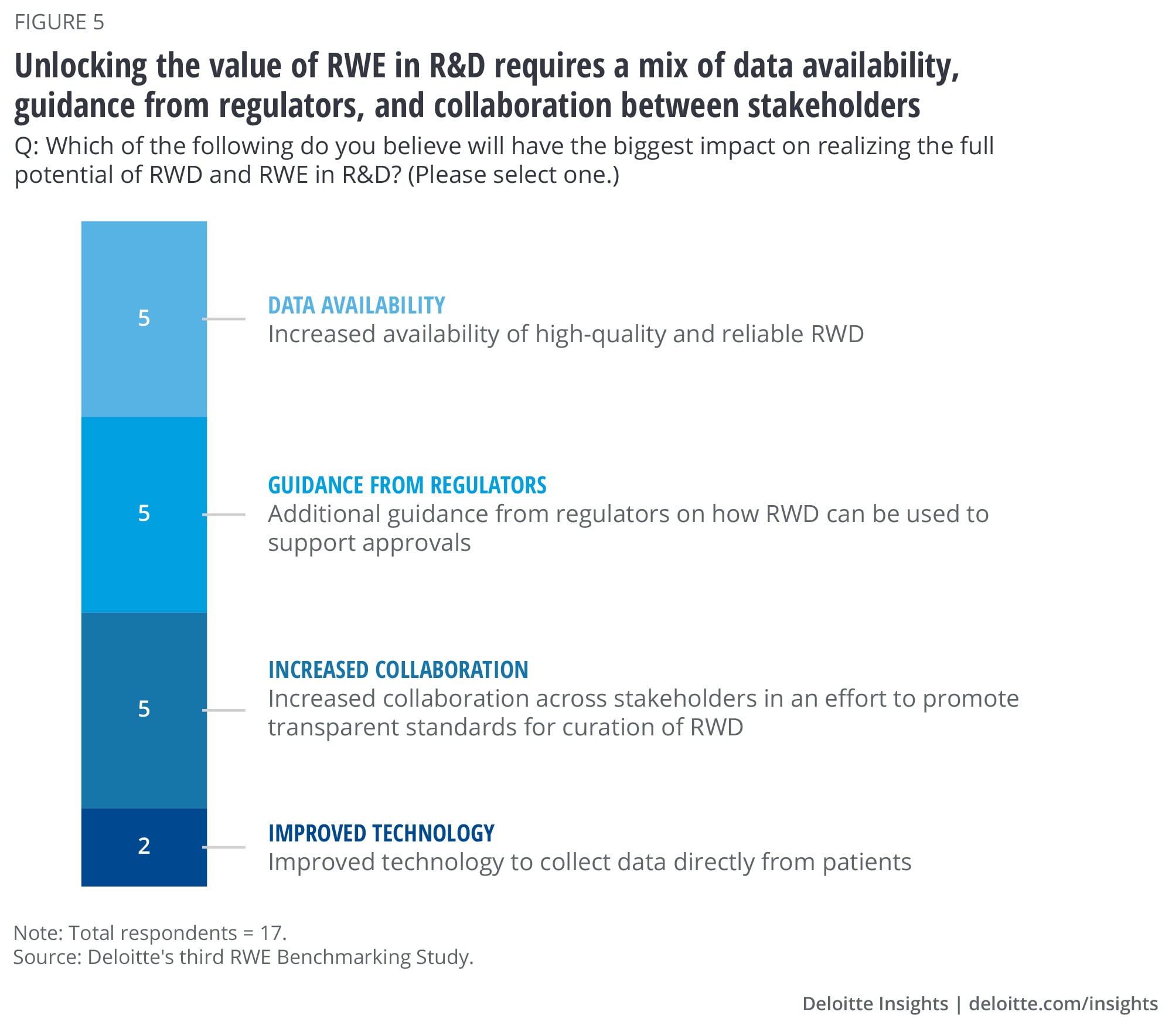
As the use of RWE continues to evolve, there are several factors that could help realize its full potential in R&D, according to survey respondents. Close to 30% of the survey respondents say there is a need for increased availability of high-quality and reliable RWD (figure 5). The same percentage also pointed to the need for collaboration to promote development of transparent standards for RWD curation. Lack of consistent data standards often result in significant labor and cost associated with data normalization and curation. Another 30% of the respondents highlighted the need for additional guidance from regulators on how RWE can be used to support product approvals. This includes guidance on use of synthetic control arms and novel trial designs as well as methodologies to assess the reliability of RWD from multiple sources and fill gaps in real-world data sets. Regulators, technology companies, pharmaceutical companies, and other industry stakeholders should collaborate on how to improve the curation of RWD and advance the use of it in the R&D setting.
Companies continue to invest in developing an internal RWE engine
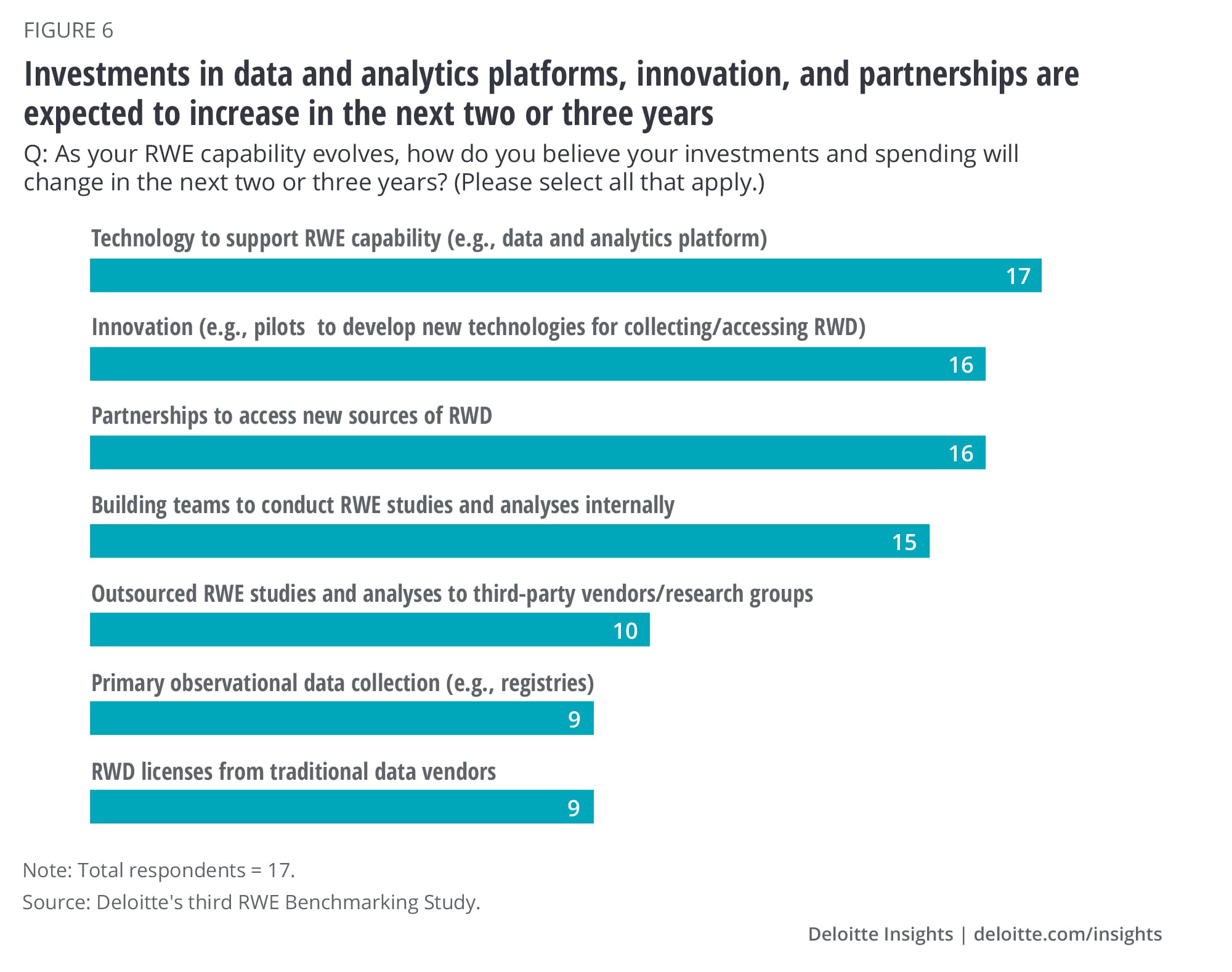
Regardless of their level of maturity, most surveyed organizations expect to continue to invest in their RWE capability across a multitude of dimensions. Spending on data and analytics platforms, gaining access to RWD, and building teams to conduct studies internally is expected to continue to grow in the next two to three years (figure 6). As spending on internal RWE capabilities continues, it is important for organizations to establish both qualitative and quantitative ways to assess the return on these investments. The time to value realization will likely vary based on organizational maturity, culture, size of investment, and focus. Setting short- and long-term key performance indicators is one way to start to measure ROI over what could be an extended time horizon.
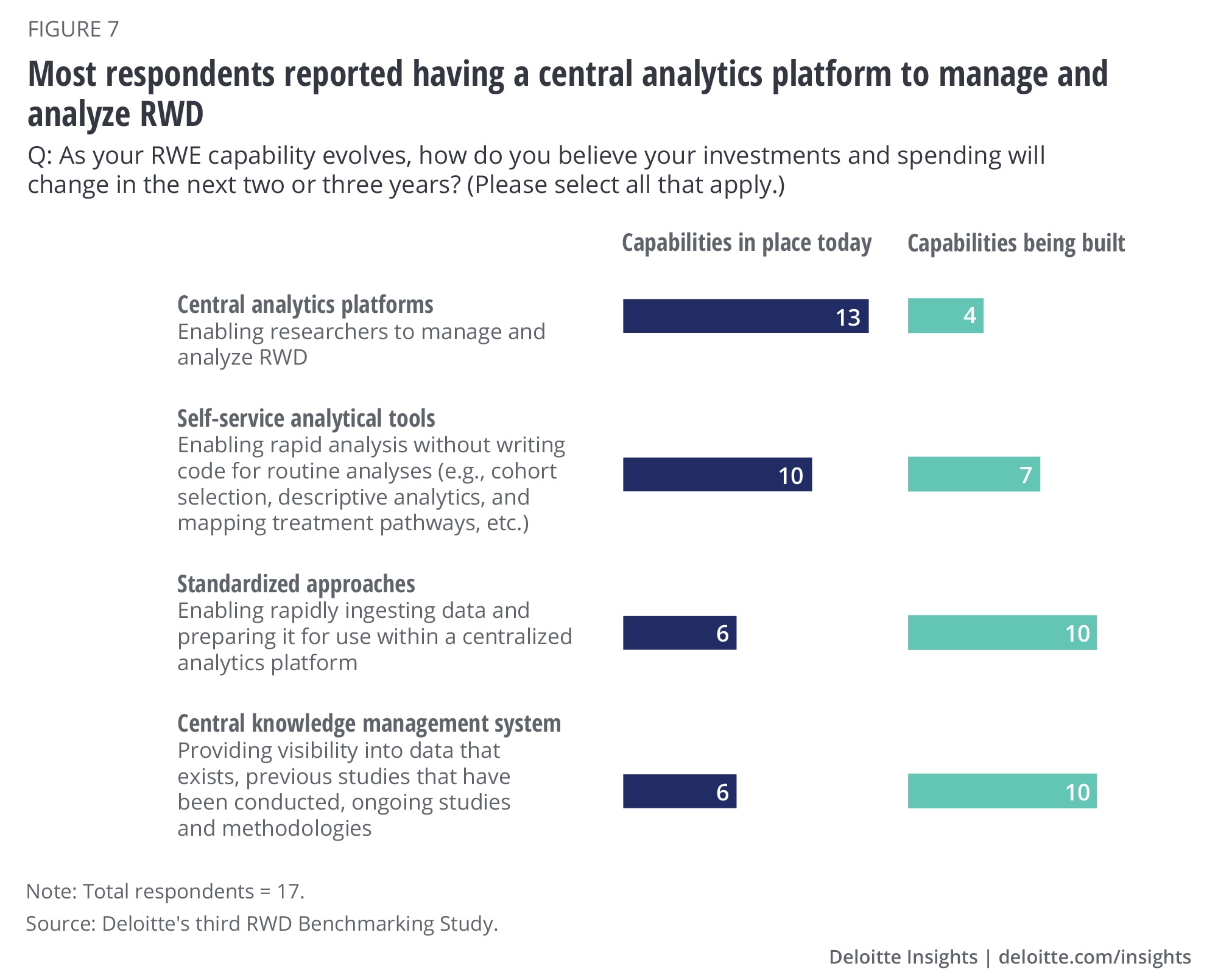
Another consistent trend with previous surveys is around key characteristics of the technology supporting RWD and RWE. There has been a clear shift to cloud-based platforms that provide tools that not only meet the needs of traditional data scientists but also those of business stakeholders and citizen data scientists. Three-quarters of respondents reported having a central analytics platform to manage and analyze RWD, of which almost all say their platforms are hosted in the cloud (figure 7). More than half also reported having in place self-service tools that allow users to conduct commonly requested analyses such as defining a cohort, generating descriptive statistics, understanding treatment pathways, or calculating incidence and prevalence of a disease on demand. These tools can enable rapid analyses without having to write computer code and can dramatically compress timelines and accelerate insight generation.
All mature companies have already established a central analytics platform (primarily cloud-based), and most have self-service tools and a centralized knowledge management and collaboration platform in place. The knowledge management and collaboration component of the platform helps to remove traditional data and analytic silos that naturally exist in many organizations, create transparency on the impact of RWD use, and enforce compliance and governance standards. This part of the platform is a key capability as it not only provides visibility into data and evidence generated across the organization and system to govern access to it, but also fosters collaboration and drives efficiencies in evidence generation. A little more than half of mature companies have also established standardized approaches to ingest RWD for analyses. This combination of technologies is likely enabling these mature companies to better leverage RWE across the enterprise and across a broader set of applications.
We anticipate that citizen data scientists and business users will become larger users of these technology platforms. When survey participants were asked about the one thing that they would like to improve about their RWE platforms, several mentioned expanding the types of tools and applications available on the platform, so that use is no longer restricted to data scientists. It also highlights the need to continuously evolve platforms in an agile way, helping ensure that the organization can meet the growing and evolving demands of the organization.
How can companies realize greater value from RWE?
RWE can fundamentally change how drugs are discovered, developed, commercialized, and reimbursed. However, realizing this value varies across industry players. All surveyed companies are increasing investment; but organizational silos, multiple fragmented technology solutions, and lack of coordination of data sources and partnerships are making it challenging to measure ROI. Mature companies have demonstrated that having a strong enterprisewide strategy and foundational technology capabilities is the first step. Here’s what both mature and developing companies can do to maximize the potential of RWE:
Invest in foundational technology capabilities: The survey results indicate that companies that have invested in a centralized cloud-based analytics capability and knowledge management platform are at an advantage compared to those that are still building this capability. Given the anticipated regulatory changes and the evidence of return on these investments that some organizations have demonstrated, making investments in these platforms and designing the new workflows and governance is a key foundational step.
Redefine your strategy and prioritize investments: Irrespective of whether companies have a mature capability or are still in the building phase, having a clear strategy can be critical. With the constantly evolving landscape, strategies should be periodically revisited. Leaders should identify near-term beneficial use cases and long-term strategic goals to help prioritize investments. It is important not to underestimate organizational change management that may be required to drive the adoption of technology and promote new ways of working across the organization.
Challenge conventional thinking: Organizations that have realized successes with RWE have provided flexibility to pilot new methods and use new data sources. RWE leaders should enable a culture of experimentation and innovation to figure out new ways of working with RWD. Quick wins from early pilots can help gain broader buy-in for new ways of working across the organization. It is important to measure and communicate the successes of these experiments and use them as a vehicle to drive cultural change.
Invest in strategic partnerships to curate research-grade/higher-quality RWD: There is a clear need for stakeholder collaboration to generate and curate higher-quality RWD and establish consistent data standards. Companies should consider partnering with health systems, health plans, patient advocacy groups, tech companies, and others in the ecosystem to leverage RWD for better patient outcomes. A collaboration model and a dedicated team to contribute to and manage these strategic partnerships are essential to ensure successful collaboration. Looking to data marketplaces can help identify potential partners.
Measure ROI: Establishing key performance indicators tied to the defined strategy can help organizations realize ROI. Our past surveys have revealed that some organizations are quantifying ROI by measuring savings through the reduction of duplicative investments in data sets or reducing overall spend with external vendors to conduct RWE studies and analyses. However, the industry should look beyond cost savings to assess value generation from RWE. This requires directly linking RWE to favorable impacts on topline sales or business objectives such as a favorable regulatory decision, speed to market, or influencing market access decisions.
Explore more on health care
-
The economic impact of COVID-19 (novel coronavirus) Article4 years ago
-
The future of health Collection
-
Hospital revenue trends Article4 years ago
-
Drug and inpatient spending lines are crossing Article4 years ago
-
Virtual health care Video