Scaling up AI across the life sciences value chain Enhancing R&D, creating efficiencies, and increasing impact
19 minute read
04 November 2020
AI is already helping improve process efficiency in the life sciences industry. The next three to five years are likely to prove AI’s value in R&D, especially in drug discovery, and across other areas of the value chain.
Executive summary
The COVID-19 pandemic has increased the focus on the use of artificial intelligence (AI) across the life sciences organization, from R&D to manufacturing, supply chain, and commercial functions. During the pandemic, company leadership and management realized that they could run many aspects of their business remotely and with digital solutions. This experience has transformed mindsets; leaders are more likely to lean into a future that lies in digital investments, data, and AI because of this experience.
Learn more
Explore the life sciences collection
Learn about Deloitte's services
Go straight to smart. Get the Deloitte Insights app
At present, the life sciences industry has only begun to scratch the surface of AI’s potential, primarily applying it to automate existing processes. By melding AI with rigorous medical and scientific knowledge, companies can do even more to leverage this technology to transform processes and achieve a competitive edge. AI has the potential to identify and validate genetic targets for drug development, design novel compounds, expedite drug development, make supply chains smarter and more responsive, and help launch and market products. We will highlight a number of these use cases in this report.
To explore the use of AI by the life sciences industry, Deloitte surveyed global leaders of life sciences (i.e., biopharma and medtech) companies about their AI investments, outcomes, and challenges. We learned that:
- More than 60% of life sciences companies spent over US$20 million on AI initiatives in 2019, and more than half expect investments in AI to increase in 2020.
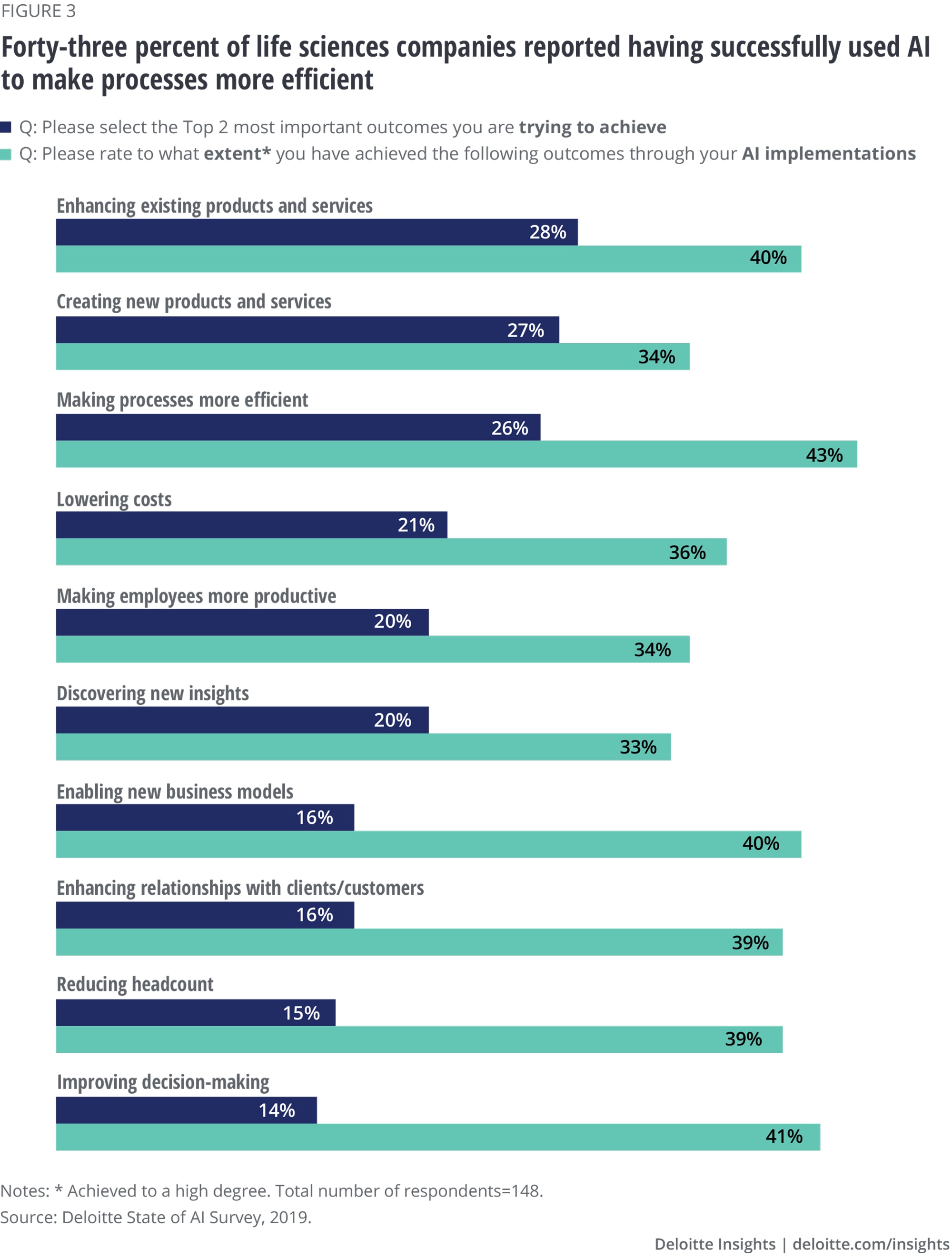
- Top outcomes life sciences companies are attempting to achieve with AI include enhancing existing products (28%), creating new products and services (27%), and making processes more efficient (22%). Most (43%) reported having used AI successfully to make processes more efficient.
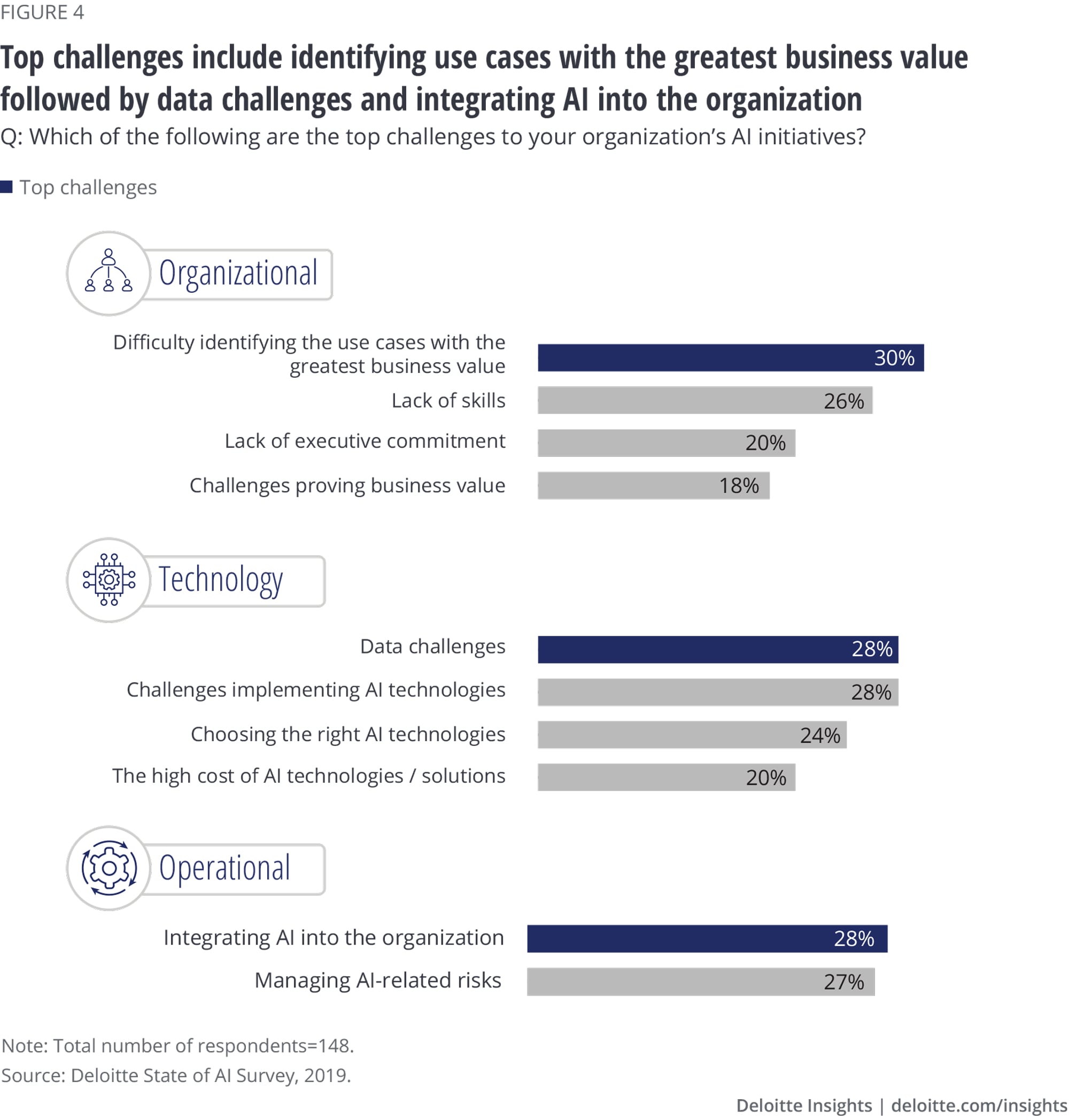
- Top challenges impacting AI initiatives include difficulty in identifying business cases with the highest value (30%), data challenges (28%), and integrating AI into the organization (28%).
AI is already showing its value in making processes more efficient and is likely to do so even more over time. The next three to five years are likely to prove AI’s value in the transformation of biopharma research and development (R&D), especially in drug discovery. In parallel, companies could pilot more AI projects and consider adopting it across other areas of the value chain.
As AI moves from a “nice to have” to a “must have,” companies and their leaders should build a vision and strategy to leverage AI, then put in place the building blocks needed to scale its use. These include the right IT infrastructure, the right talent and skill sets, and creating ecosystems and alliances to access or build AI capabilities. Creating frameworks and protocols to manage AI-related risk as well as audit AI systems for bias and transparency will also become essential to meet compliance and regulatory requirements.
Introduction
As COVID-19 unfolded and its global impact became clear, biopharma companies quickly turned to AI to identify candidates for vaccines and drugs, conduct virtual trials, and build resilience and adaptability in manufacturing and supply chain capabilities. Projects that may have been planned for a few years out were suddenly being implemented immediately to address the pandemic. This pivotal movement pushed AI into the spotlight. As our survey results indicate, life sciences companies’ investments in AI are expected to continue to grow in 2020. Coupled with an explosion in the availability of health care data, advances in cognitive computing, and machine learning techniques, the use of AI is positioned to expand across the biopharma value chain, from molecule to market.
In Deloitte’s longer-term vision of the future of health, AI will not only transform life sciences but also affect health care more broadly. AI-supported precision medicine, digital therapeutics, and clinical diagnostic and treatment support will lead to better health outcomes (for more see sidebar, “AI and the future of health”). Preparing for this future requires life sciences companies to begin acting today.
AI and the future of health
In the future of health, AI will enable major scientific breakthroughs, accelerating the creation of new therapies and vaccines to fight diseases. AI-enabled digital therapeutics and personalized recommendations will empower consumers to prevent health issues from developing. AI-generated insights will influence diagnosis and treatment choices, leading to safer and more effective treatments. Additionally, intelligent manufacturing and supply chain solutions will ensure the right treatments and interventions are delivered at the exact moment needed by the patient.
An enterprisewide approach to AI use
Deloitte’s research and expertise have helped us identify how AI can be applied across the biopharma value chain from molecule to market. AI has the potential to identify and validate genetic targets for drug development, design novel compounds, expedite drug development, make supply chains smarter and more responsive, and help launch and market products.
While implementing AI in each of these areas can drive new efficiencies and insights, AI will likely be most effective when deployed strategically across the entire enterprise. With coordination between functions, a governance structure that prioritizes business needs, and a standardized process to address cyber and compliance issues, biopharma companies will be better able to reap larger benefits from their AI investments as compared to taking a piecemeal, siloed approach.
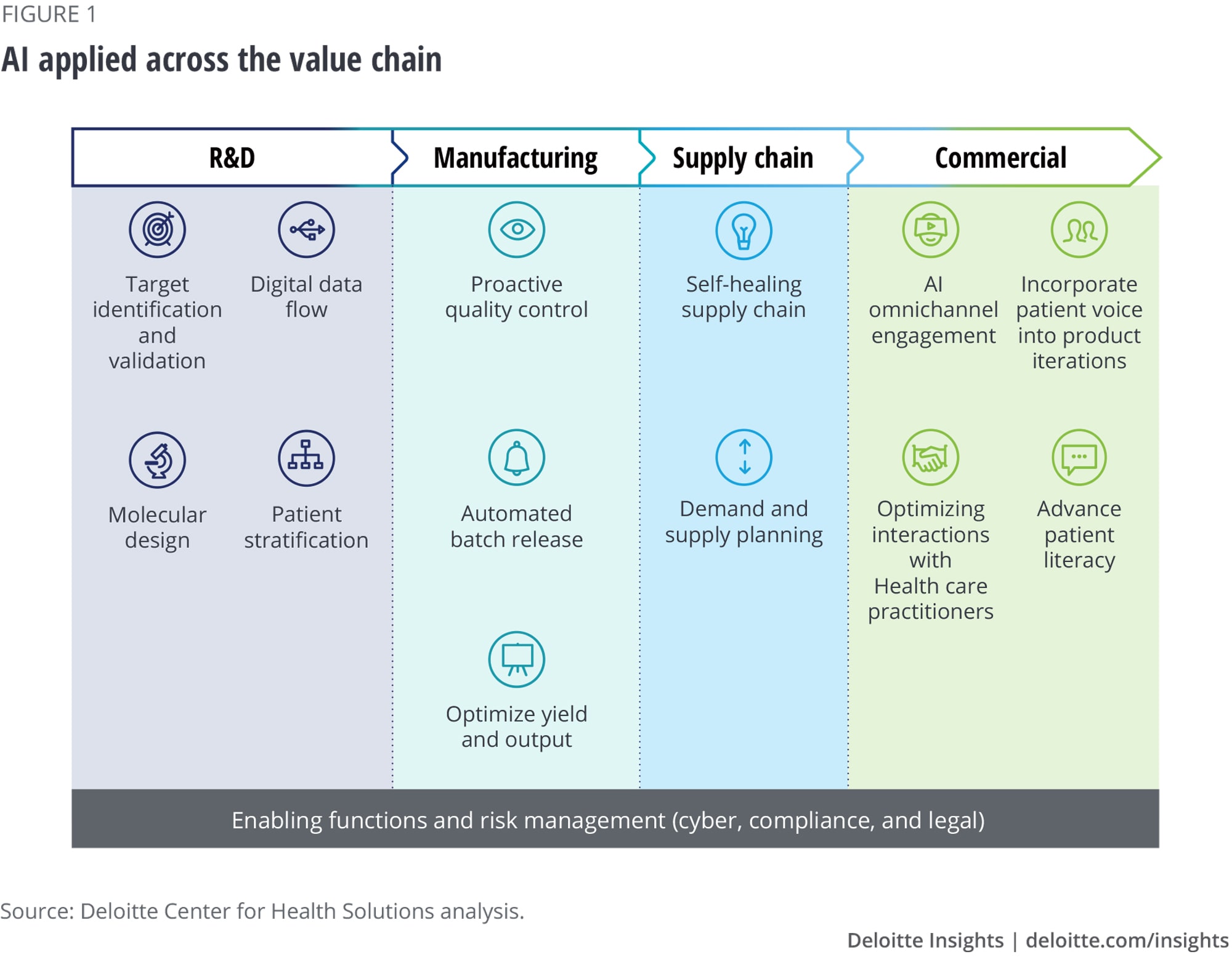
Research and development
Drug discovery involves a series of laborious, manual, and capital-intensive processes that can be automated or augmented through the use of AI. Biopharma companies are already beginning to experiment with AI to speed up drug discovery activities (see case study 1). In the next five years, companies could increasingly use AI models to identify and validate targets, design molecules, synthesize and test these molecules in silico, and feed data back into these models to improve their predictive capabilities. This could vastly accelerate the traditionally slow drug discovery process.
The growing abundance of genotypic and phenotypic data has created a need for techniques that can transform and expedite target identification and validation. AI imaging capabilities can detect cell morphology changes that humans cannot see through a microscope. AI applied to knowledge graphs could help understand complex relationships between compounds, genes, diseases, and proteins. Validating targets through functional genomics experiments could make extensive use of AI to interpret phenotypic changes in cells via imaging, and integrate disparate readouts such as expression assays, cell images, and epigenetics (noncoding changes that affect expression). To prevent expensive failures in late-stage drug development, generating genetic evidence for target identification will become an increasingly important strategic priority.1
In the molecular design space, several startups are already pioneering the use of generative modeling for small molecule design and protein engineering through partnerships with large biopharma companies. At the same time, biopharma companies are also building their own capabilities to augment medicinal chemists in their design work. Over the next five years, generative modeling2 could become an important part of computational chemistry toolkits, enabling companies to explore novel spaces and broaden the pool of drug candidates.
Today, creating trial artifacts (such as case report forms and study reports) requires manually inputting data into multiple systems. This leads to inconsistencies and errors and the need for rework, slowing down trial execution. AI-driven digital data flow solutions could integrate trial data from multiple source systems and documents to create standardized digital data elements for transmission to downstream systems. These data elements can then be used to auto-populate required reports and analyses and generate content for trial artifact creation. Some organizations are already exploring the feasibility of using AI to better manage clinical trial data (see case study 2).
Targeting patient populations to demonstrate efficacy will become an important aspect of clinical trial design over the next decade, speeding up the development process and reducing the number of failures. AI will be increasingly leveraged to normalize data from different platforms (e.g., gene expression data) and integrate multiple data points (genotypic, imaging, clinical records, and epidemiological) for patient stratification and identification of patients most likely to improve the probability of trial success. AI-enabled companion diagnostics tools (e.g., those used to screen for biomarkers) could be used to better understand the profile of response during clinical research and then give clinical decision support upon the approval/commercialization of the medicine.
Case study 1: Expediting drug discovery with AI3
In January 2020, Sumitomo Dainippon Pharma announced the start-up of a Phase 1 trial for a candidate to treat obsessive-compulsive disorder created using AI. This involved using Exscientia’s AI drug discovery platform to identify a serotonin 5HT1A receptor agonist that could be effective in treating patients with obsessive-compulsive disorder. Leveraging AI enabled identifying this molecule within 12 months as compared to the several years it takes using conventional research techniques.
Case study 2: Digital data flow across the clinical trial4
A large biopharma company organized a hackathon to test the feasibility of using AI to automate the study startup process with the goal of reducing trial cycle times and costs. The hackathon explored how AI could process and interpret data in unstructured documents (e.g., study protocol), identify discrepancies in manually entered trial data, and digitalize data elements in key documents so that they could be transferred to downstream systems without manual effort.
Manufacturing
Fixing manufacturing issues today requires laborious manual intervention to access multiple systems, with action taken only after problems occur. Applying AI to manufacturing data could help predict process bottlenecks, identify quality control issues, and proactively suggest corrective actions. Applying AI technologies can help reduce manual oversight in manufacturing operations and allow tighter control of quality and operating costs through:
- Proactive quality control by applying machine learning (ML) to assess manufacturing data from multiple batches, product lines to identify process discrepancies, and predict quality issues. This can direct staff to investigate only those batches most likely to have quality issues, saving time and resources.
- Coupling AI with robotic process automation (RPA) to automate batch release and documentation can generate a comprehensive and auditable data trail to meet regulatory and compliance requirements.
- AI-driven simulations and modeling that assess various parameters during the manufacturing process to enable corrective actions to optimize yield and output. Our research suggests AI is uniquely suited to assist in improving antibody bioreactor productivity. Applied to imaging data of modified cell cultures, AI could help predict which cell lines are likely to fail early and improve the productivity of the bioreactor.
- AI-enabled predictive maintenance activity could reduce machine downtime, production disruptions, and loss of expensive API materials. This will help reduce operating costs.
Enabling these applications calls for investing in aggregating data from multiple manufacturing systems and thoughtful placement of sensors across the manufacturing floor. Some early movers are already beginning to report benefits from applying AI to manufacturing activities (see case study 3).
Case study 3: Optimizing manufacturing activities using AI
To improve current manufacturing processes, a biopharma company applied analytics and neural networks technologies to manufacturing data at one of its sites in the United Kingdom. This improved manufacturing line speeds by 21%, cut downtime, and delivered an overall equipment effectiveness improvement of 10%. Additionally, the company was able to improve detection of quality defects and optimize machine throughput. The company is planning to apply deep-learning image recognition to detect quality defects at the site.5
Supply chain
Today, many companies respond reactively to supply chain disruptions and are slow to adjust inventory and production levels. With the pandemic exposing the fragility of the biopharma supply chains and new product types (e.g., cell therapies) with complex logistical requirements entering the market, there is an urgent need to improve supply chain visibility and adaptability.
Early movers are already beginning to tap the potential of AI to automate demand and supply planning (see case study 4). AI could automate analysis of aggregated manufacturing, supply chain, and marketing data to predict demand and supply, recommend the next best action to supply chain operators, and even autonomously perform certain activities. Such self-healing AI supply chain solutions could improve the ability to dynamically respond to changes in market demand and supply availability, enable quick recovery from disruptions, and improve decision-making related to product distribution and new product introductions.
AI will also become an important enabler for the industry to transition away from the use of linear supply chains to dynamic digital supply networks (DSNs). DSNs leverage digital technologies such as sensors, RPA, blockchain, advanced analytics, and AI to collate data from multiple sources and locations in real time to optimize production and distribution (for more see Deloitte’s publications on digital supply networks and intelligent supply chain).
Case study 4: ML to automate forecasting6
Merck KGaA has implemented an AI-based system to automate demand forecasting for supply chain planning. The company has been applying ML to data from its enterprise resource planning (ERP) system to accurately forecast the demand for its products in terms of both quantity and location and suggest changes to inventory and production levels. By using this technology at scale, Merck improved the forecast accuracy of 90% of its products.
Commercial
Current biopharma consumer engagement strategies across channels (TV, social media, print, web, sales representatives) tend to address broad groups rather than precisely target customers based on individual needs and preferences. This often results in irrelevant and unwanted engagement, leading to low returns on marketing spending.
Omnichannel AI engagement solutions applied to analyze multiple data sources (including socioeconomic, demographic, location, medical history, and sales data) can predict how, when, and with what types of messages to engage with patient and health care practitioners (HCPs). This could enable personalized just-in-time content such as suggestions on a patient’s smartphone when they enter a pharmacy or read a WebMD article. While engaging HCPs, omnichannel AI could provide recommendations to marketing and sales representatives on next best actions, channels, and personalized content to engage HCPs. A few companies are already experimenting with AI to optimize interactions with HCPs (see case study 5).
Beyond marketing, AI can be used to analyze patient complaints, medical inquiries, and social media data to incorporate the voice of the patient into product iterations (see case study 6). Natural language processing (NLP) applied to structured and unstructured data across silos can create a “voice of the market” feedback loop for key business areas such as medical affairs, market access, sales, and brand teams. Our research also suggests conversational AI technologies are being used increasingly to advance patient literacy by equipping patients with de-jargonized insights to help them understand treatment options and trade-offs.
Case study 5: Augmenting sales representatives’ interactions with physician7
Biogen created a custom internal search engine that leverages NLP to help sales representatives to quickly find answers to physicians’ questions on company products. The system replaced assembled-over-the-years FAQs, text-heavy documents, and educational material that sales representatives need to comb through for answers. The use of NLP enabled sales representatives to type in the exact words of the physician and get an answer to a query quickly.
Case study 6: Bringing the patient’s voice into product iterations
A life sciences company used NLP to aggregate and analyze consumer complaints, social media sentiment, and adverse event data on a newly launched injection system for plaque psoriasis and bipolar disorder. From this AI- enabled analysis, the manufacturer discovered that consumers were confused by device instructions and were incorrectly using the injection system. In response, the manufacturer created a more intuitive syringe system with simpler user instructions and redistributed the product to the market.
AI in risk management
Companies are increasingly applying AI to help with identifying, monitoring, and addressing risks to support compliance and protect against cyber threats.
Traditionally, compliance functions have taken a risk-based approach to set priorities and then monitor activities with the highest potential for compliance risk. As regulatory pressures mount to proactively monitor risk, compliance staff cannot keep up with the vast amount of information compliance programs are expected to monitor. Applying AI solutions can help proactively identify anomalies, prioritize compliance risks, and improve the efficiency of compliance staff and activities (see case study 7). Automating the processing of adverse events is one area where biopharma companies are increasingly applying AI to handle the growing volume of adverse event data (see case study 8).
Case study 7: Improving the efficiency of compliance functions8
A biopharma company deployed an NLP tool for initial email screening to identify potential risks. This reduced the number of emails compliance officers needed to review from 2,500 to 20. Officers could then spend time investigating those 20 emails and analyzing them to find true risk to the business as well as perform employee retraining.
Case study 8: Automating adverse event reporting to improve patient safety and compliance
Using Deloitte’s ConvergeHEALTH’s cognitive case processing algorithm, a large biopharma company automated the processing of adverse event data from patients, health care professionals, and regulatory agencies. The solution applies ML and NLP to automate routine cases and route exceptional cases to experts for targeted adjudication, and then learns how to handle similar cases to improve efficiencies.
Applying the solution has improved the quality and consistency of safety information and freed resources to focus on a deeper understanding of the safety profile of the company’s products. The solution has reduced case processing time by 50–60% per case, which has resulted in 65–75% savings per year in case-processing costs.
Biopharma companies routinely interact with HCPs through contractual arrangements such as paying a standard fee for participation in an event or provision of services. These contracts are subject to regulatory and legislative scrutiny. Companies could increasingly use software bots, NLP, and machine learning to review contracts to ensure they comply with internal norms and external regulations. Current systems can accurately and confidently make “approve/reject” decisions for more than 70% of contracts.
AI solutions are also being used as part of cyber strategy.9 Modern cyberattacks can circumvent traditional, rule-based security controls by learning detection rules. Smart cyber technologies will be increasingly needed to protect the growing volume of data biopharma companies own and have access to. AI-based approaches can improve threat intelligence and prediction, and enable faster attack detection and response, augmenting efforts by cybersecurity experts (for more, see Deloitte’s publication on Smart Cyber: How AI can manage cyber risk).10
Survey findings
Deloitte surveyed nearly 150 executives in the life sciences industry to understand how organizations are adopting, benefiting from, and managing AI. In addition to the case studies presented above, these survey findings provided a macro-level view on AI uptake across the industry.
AI investments by life sciences organizations are expected to increase
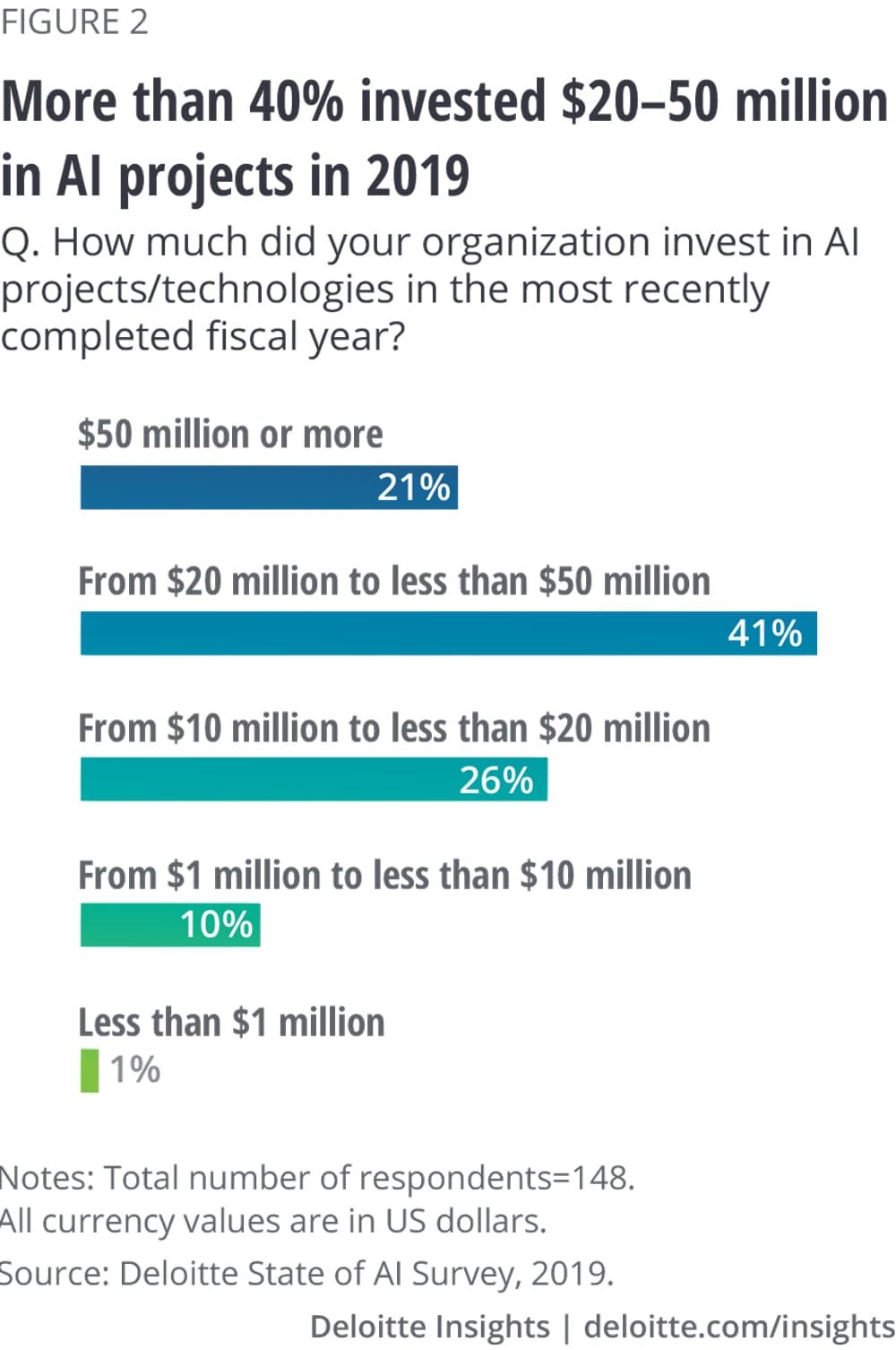
AI investments by life sciences organizations varied in 2019. More than 40% of survey respondents reported their organizations invested US$20–50 million, while 21% invested more than US$50 million on AI initiatives. More than half of the survey respondents expected investments in AI to continue to increase in 2020. We believe investments in R&D are likely to continue to accelerate because of its central role in developing new products and intellectual property. As mentioned earlier, we also expect investment in AI to grow across the value chain as more executives recognize how critical digital transformation is to their companies’ future.
Unlike ERP systems that begin showing returns in a few months or a year, AI implementations require time to ramp up and provide benefits; 46% of respondents say it takes longer than expected to receive payback from investments in AI initiatives. Training an AI model is an ongoing process, and as the model is trained over time, returns on investment gradually increase. Executives therefore should account for model learning while calculating payback periods on AI projects.
Research methodology
Between October and December 2019, Deloitte’s Center for TMT surveyed 2,737 IT and line-of-business executives on AI investments by industry. The Deloitte Center for Health Solutions then analyzed 148 survey responses from life sciences executives to understand their organizations’ current and anticipated investments, top priorities, and AI risks and concerns. Survey respondents included a mix of executives from biopharma and medtech organizations across the globe.
We also interviewed industry executives and experts to better understand their views and get their perspectives on the survey results.
COVID-19 is accelerating focus and investments in AI
Interviewed executives believe the pandemic will act as a catalyst for the industry to adopt and invest in AI on a larger scale. Most see COVID-19 as providing the opportunity and rationale to address long-standing problems and bring executive attention and support to deploy automation and AI to solve them.
The rapid spread of COVID-19 has forced companies to run trials remotely and increased focus on using AI to accelerate drug discovery. Our interviewees pointed out that the business impact of not having an agile supply chain has led to increased awareness and urgency to deploy AI to build supply chain resiliency. Others said new internal groups were beginning to form and funding is now available to explore more use cases for AI.
Top priorities include enhancing existing products and services, creating new products and services, and increasing process efficiency
Forty-three percent of survey respondents reported that AI implementations resulted in making processes more efficient. When asked about the outcomes companies are trying to achieve through AI use, surveyed executives cited enhancing existing products and services (28%) as a top priority. This was closely followed by creating new products and services (27%) and increasing process efficiencies (26%). While we agree that AI can enhance existing products and make business more efficient, we expect AI use in creating new products to grow as companies embrace more advanced AI applications and make it a central part of their business transformation.
Top challenges include identifying high-value use cases, AI integration, and data and risk issues
Survey respondents pointed to multiple challenges associated with AI implementations, including poor-quality data, siloed data systems and integrating AI into legacy systems, and risk management. Top challenges for life sciences companies include identifying use cases with the greatest business value (30%), integrating AI into the organization (28%), and data challenges (28%). Our interviewees also said finding talent with industry expertise and AI skill sets is difficult.
Surveyed respondents also pointed to the lack of explainability of AI algorithms or the AI black box issue as a major concern. To address this, there is a need to demonstrate that AI during its learning process has not deviated from providing a valid or intended output. This will help not only gain regulatory approval to expand the use of AI but also lead to greater trust in and use of AI systems across the industry.
Strategies to reduce bias and increase transparency
Embedding AI into processes and workflows presents multiple risks to life sciences companies, including variability of output, biases, cybersecurity vulnerabilities, and liability for the decisions and actions that AI takes. To get to the ensemble of the AI algorithm best suited for the organization, there is a need to not only ensure accuracy of output but also address issues related to transparency and bias. This typically requires companies to:
Test: As AI algorithms can produce varying outputs each time they learn, they need to be tested differently from other software. Testing AI models for accuracy should ensure the model is stable and produces consistent results given the same set of test data.
Reduce bias: AI models are only as good and unbiased as the data used to train them. To minimize data biases, companies should put in place a strategy to ensure standardization, equity, and fairness in the underlying data. Engaging data scientists to understand the causes of data bias and how to best mitigate the data risk is critical. Additionally, organizations should ensure end users are trained on how to interpret and react to AI outputs while recognizing biases before taking the next action.
Audit and monitor: Companies must continuously monitor AI for errors or biases that were not apparent in training or that develop due to a changing environment or new data. Auditing an AI model requires being able to trace back how it arrived at a particular decision. For instance, in the case of the adverse event processing tool (see case study 6), the algorithm should be able to point out the exact phrase it used to arrive at a decision.
As companies incorporate AI into their business practice, regulators are likely to update their policies and standards to include AI. In the interim, companies need to balance using AI to improve their own compliance processes while staying in compliance with changing regulations.
Call to action: Taking AI to the next level
As AI moves from a “nice to have” to a “must have,” companies should consider three sets of issues. First, company leaders should define their vision and strategy for how they want to take advantage of and what they expect to get from investments in data, analytics, and AI. To carry out that strategy, companies need to assemble the building blocks to ensure success. Once these are in place, scaling up through internal investments or partnerships can help companies go from short-term to longer-term success in the market.
Vision and strategy
- Leadership: A top-level leader can bring visibility, cohesiveness, and connectivity to AI use across the organization and articulate a vision that is more than a sum of pilots and experiments. Such a leader could act as a champion for AI use cases, communicating successes from pilots, and garnering funding to upscale AI implementations.
- The North Star: Companies need to identify where, when, and how they intend to embed AI into business operations and strategies. This could enable defining a North Star for AI and then building a vision or strategy around it.
Assembling the building blocks
- Technology and data: AI is not something that is added as a module to existing systems. It is a connecting thread that has to work cohesively with the existing technological backbone of an organization, be it an ERP system or other digital technologies. AI algorithms are only as good as the data they are trained on; so companies should invest in developing data standards and ways to analyze diverse metadata.
- Technology architecture: Upscaling AI use requires investing in the right IT architecture and infrastructure assets. Organizations should identify what technology capabilities, data, and systems are needed to upscale the use of AI and determine how existing data architecture needs to be modified.
- Ecosystems and alliances: Forming AI ecosystems and alliances to work more closely with AI start-ups and a broad group of partners can enable life sciences companies to either build or gain access to AI technologies.
- Talent: Biopharma companies are already competing for scarce data scientist talent with technology companies; sometimes different divisions within a biopharma company are vying with each other for the same talent and skill sets. A talent strategy could include understanding skill availability within the company and reskilling and upskilling the existing workforce. Companies can also source talent from vendors, ecosystem partners, and third parties.
- Align risk management efforts, audit, and test: AI-related risk management should be integrated with the organization’s broader risk approach, given that it is using data and technologies that are part of existing systems. Teams working on AI need training in all the elements of risk—protecting against cyber threats, fraud and abuse, and safeguarding intellectual property. Companies should build protocols to audit and test AI systems regularly to identify biases and ensure business and scientific leaders can understand AI-based recommendations.
Scaling up
- Building an AI center of excellence (CoE): Setting up an AI CoE can help turn a vision for AI use into a scaled strategy. The CoE can broker lessons learned from pilots and project implementation as well as lead the development of a governance framework to manage AI-related risk across the organization.
- Pilot projects: Establishing a first set of use cases to pilot and then being willing to fail fast and learn quickly can help life sciences organizations understand what works for their company. From there, they can scale projects across the organization.
- Governance framework: As organizations begin scaling AI, governance frameworks will be important to ensure AI addresses business priorities. Additionally, AI should be fair and unbiased, transparent and explainable, robust and reliable, respectful of privacy, responsible and accountable as well as safe and secure. Deloitte’s Trustworthy AI Framework can help ensure the transparent and ethical use of AI across the organization.
© 2021. See Terms of Use for more information.
Learn more about technology in life sciences
-
The future of medtech Video4 years ago
-
Medtech leaders prioritize technology and consumers Article4 years ago
-
New payment models in medtech Article5 years ago











