Intelligent drug supply chain Creating value from AI
8 minute read
15 June 2020
Protecting biopharma supply chains is a priority for companies ensuring access to lifesaving products. This report examines the rationale for transforming the supply chain and the role that AI can play in its digital transformation.
Our Intelligent biopharma series explores the ways artificial intelligence (AI) can impact the biopharma value chain. The first two reports, Intelligent drug discovery and Intelligent clinical trials, highlight the potential of AI to accelerate the development of new drugs. This report explores the digital transformation of the biopharma supply chain and how AI can help improve value and manage risks more effectively. Given the unprecedented challenges due to the COVID-19 pandemic, the report also considers the role that AI can play in helping the supply chain to respond, recover and thrive.
The rationale for transforming the biopharma supply chain
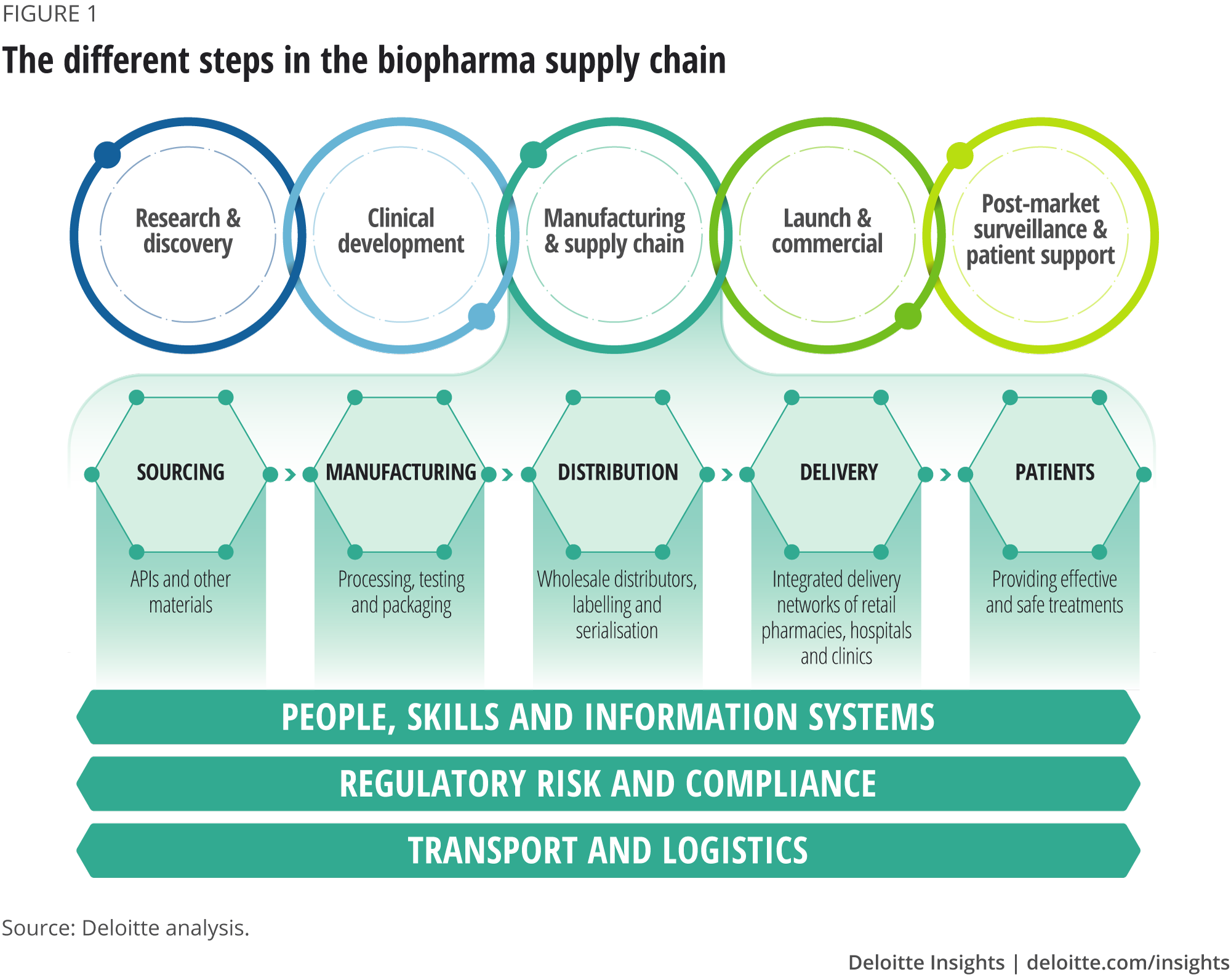
The biopharma supply chain involves a complex set of steps that are required to produce a drug, from sourcing and supply of materials, through manufacturing and distribution, to delivery to the consumer. This forms a golden thread between the discovery of new therapies and patients receiving them (figure 1).1
Learn more
Download the complete PDF and get access to eight case studies
Explore the AI in Biopharma collection
Explore the Life Sciences collection
Explore the AI & cognitive technologies collection
Learn about Deloitte's services
Go straight to smart. Get the Deloitte Insights app
The need for digital transformation of the biopharma supply chain has never been more pressing. Biopharma supply chains create inherent resilience risks for corporations and governments alike. Supply chains must also meet the expectations of a complex range of stakeholders, comprising multiple payers, health care providers, national and international regulators, and patients with complex and varied needs, both within and across different countries.
Protecting the biopharma supply chains is a high priority for governments, as ensuring access to lifesaving and life-enhancing products is vital for the health and well-being of their populations. Consequently, intelligent and insightful monitoring and management of the supply chain is imperative. While life sciences companies have explored the opportunities that digital technologies offer, many are yet to make consistent, sustained and bold moves to take advantage of the new capabilities.2
How AI can augment supply chain transformation
A huge amount of data is generated across the biopharma supply chain but historically has been underutilised. Using AI to process these data will be critical to supporting real-time decision making, orchestrating operational efficiency and, ultimately, creating a cost-effective, near autonomous and thriving supply chain. Deloitte has identified five critical areas and processes of the supply chain where AI is likely to have the highest impact.
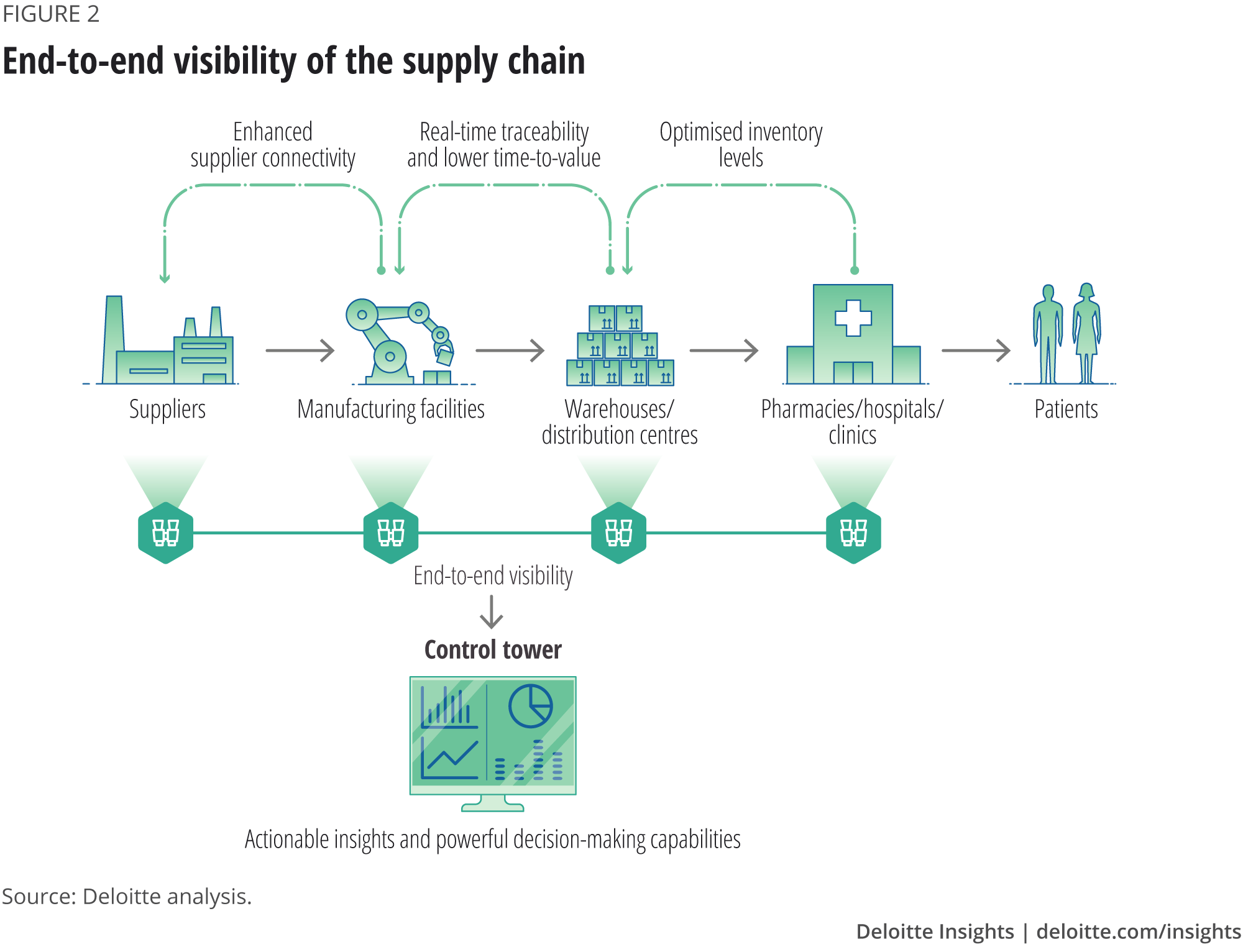
- End-to-end visibility
Supply chain visibility means having access to data relating to every transaction and demand trigger, across every step and tier and all the logistics movements in between. The concept can be realised through supply chain control towers that function as centralised hubs that collect information from disparate systems to be used for monitoring, auditing and to generate insights (figure 2). 3 - Demand forecasting, inventory management and logistics
Accurately adjusted inventory levels are needed if the value of the supply chain is to be unlocked and, importantly, patients are to obtain timely, reliable access to their therapies.4 Leveraging advanced, intelligent technologies, including predictive analytics, can help track the state of the drugs throughout the supply chain and take proactive and timely interventions when any issue arises.5 - Intelligent automation enabling Industry 4.0 and the Internet of Things
Digitalisation and intelligent process automation (IPA) can help companies establish cost-effective, reliable and robust processes that are coordinated across the supply chain.6 IPA can mimic human interaction and make advanced decisions based on the outputs of robotic inputs, minimising human errors, improving performance metrics and generating strategic insights.7,8 - Optimising predictive maintenance
Business disruption, due to a compliance, quality or safety-related issue, is a common challenge for biopharma companies. This can be minimised using AI-enabled predictive maintenance that provides insights about operations and equipment performance, including forecasting faults or other issues, to improve operational effectiveness, including machine uptime.9 - Protecting the integrity of the supply chain
Counterfeit or substandard drugs are a problem for the industry, as well as for international health organisations and society in general.10 However, for biopharma the importance of supply chain integrity goes beyond counterfeit products, as key product types need chain of identity and chain of custody. To tackle this, companies are investing in blockchain and AI technologies to improve security, transparency and traceability.11,12
AI’s role in helping supply chains respond, recover and thrive after COVID-19
During 2020, the COVID-19 pandemic has affected most aspects of biopharma’s global supply chain, from sourcing raw materials to distributing finished products. With much of the global population in quarantine, plant closures and supply shortages across the extended supply network are leading to significant global supply chain disruption.13,14
Deloitte considers that business recovery comprises three phases: respond, recover and thrive (figure 3). As companies attempt to respond and create agile, resilient businesses, the role of governance, risk management and compliance is increasingly important. Planning early for recovery, including deploying intelligent risk sensing, can help enhance the resilience of a recovery programme and set the foundation for companies to thrive after the pandemic is over.15
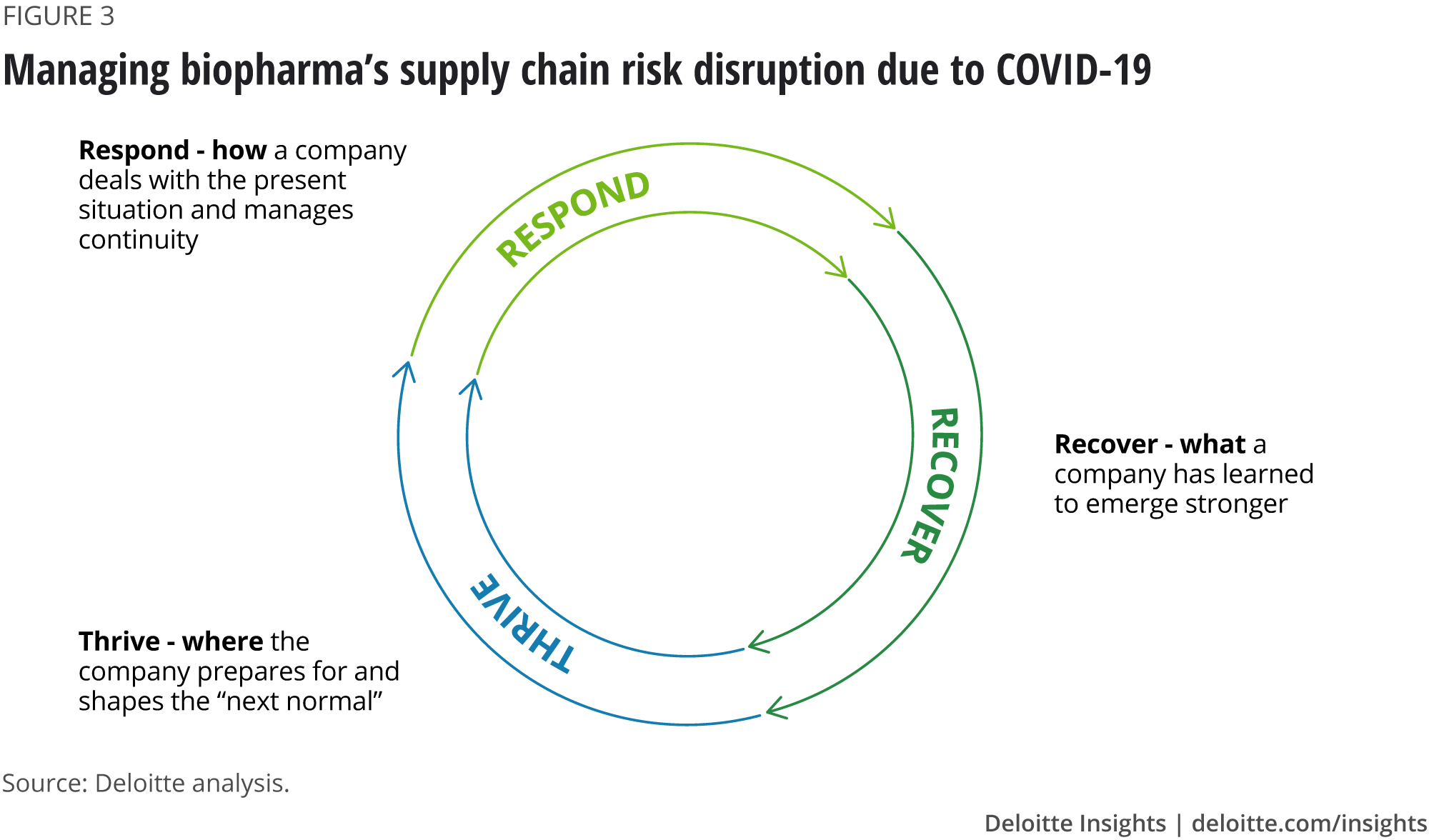
Companies that have developed and implemented supply chain risk management and digital transformation strategies alongside business continuity strategies will be better prepared to mitigate the impact of disruptions like COVID-19 and continue to thrive.16 Good practices are emerging, including innovative approaches to business continuity, using advances in IT, analytics and digital technologies. Capturing, sharing and learning from these practises will be crucial in consolidating the recovery. AI-enabled business processes should help biopharma companies respond, recover and thrive more effectively to current and future disruptions.
Roadmap for implementing an intelligent supply chain
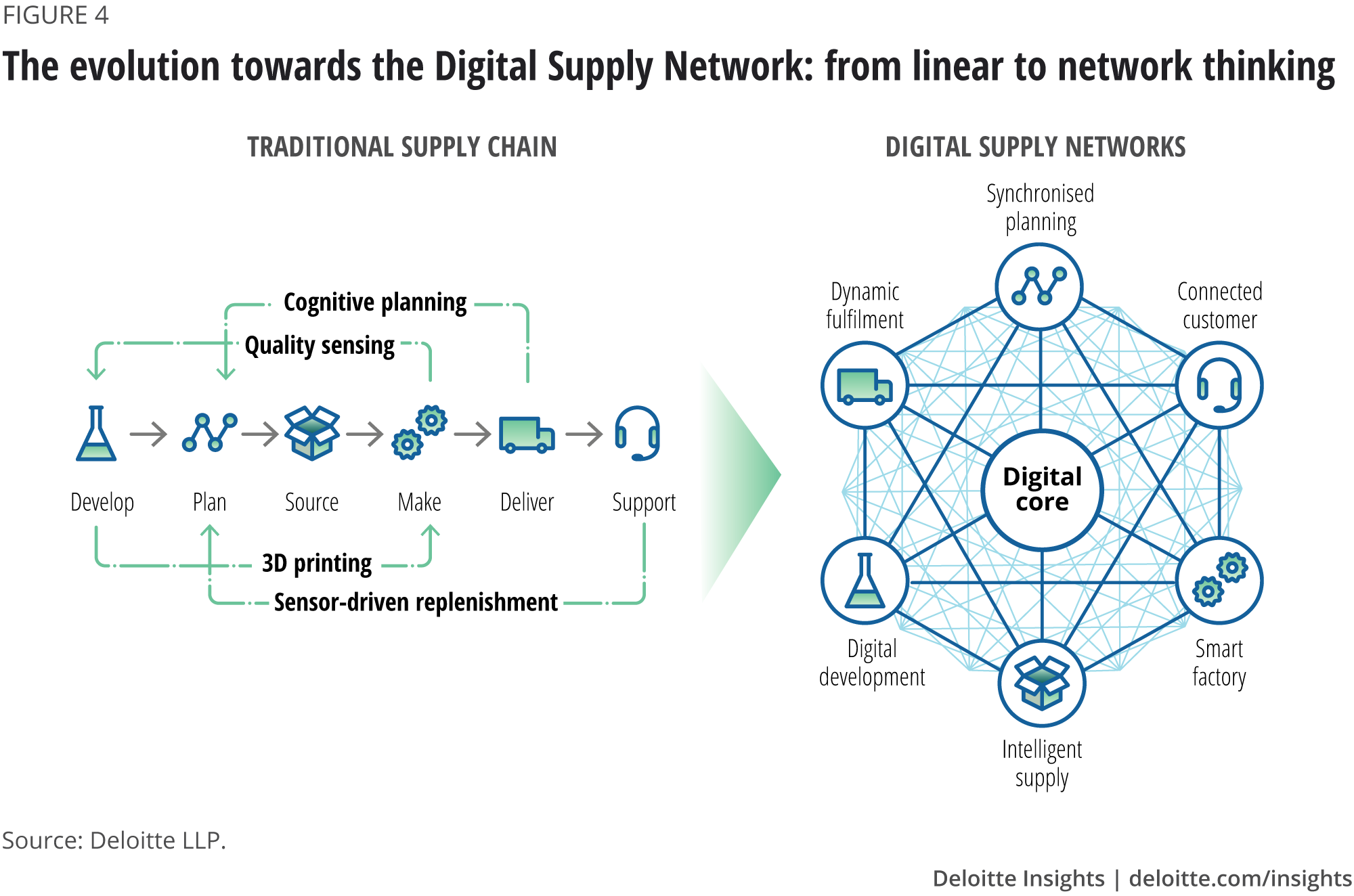
In the next few years, AI will transform the operating models of the industry. However, full digitalisation takes time and strategic thinking, and involves a fundamental shift from linear supply chains to dynamic, interconnected and open AI-enabled digital supply networks (DSNs) (figure 4).
The approach of manufacturers across industries to help integrate new digital technologies involves three stages:
- Start small: To quickly prove the value of AI-enabled solutions, companies should identify and prioritise small proof of value pilots to achieve quick wins that build confidence and buy-in.
- Scale fast: Once an implementation is proven, companies should scale the project across the supply chain.
- Think big: Successful deployment focuses on a value-driven approach for innovation and applied throughout the supply chain. There are significant benefits that companies can realise from implementing digital supply networks and having data and information immediately available to all parts of the network. 17
The following AI technology roadmap can support biopharma in successfully developing a digital transformation strategy (figure 5).
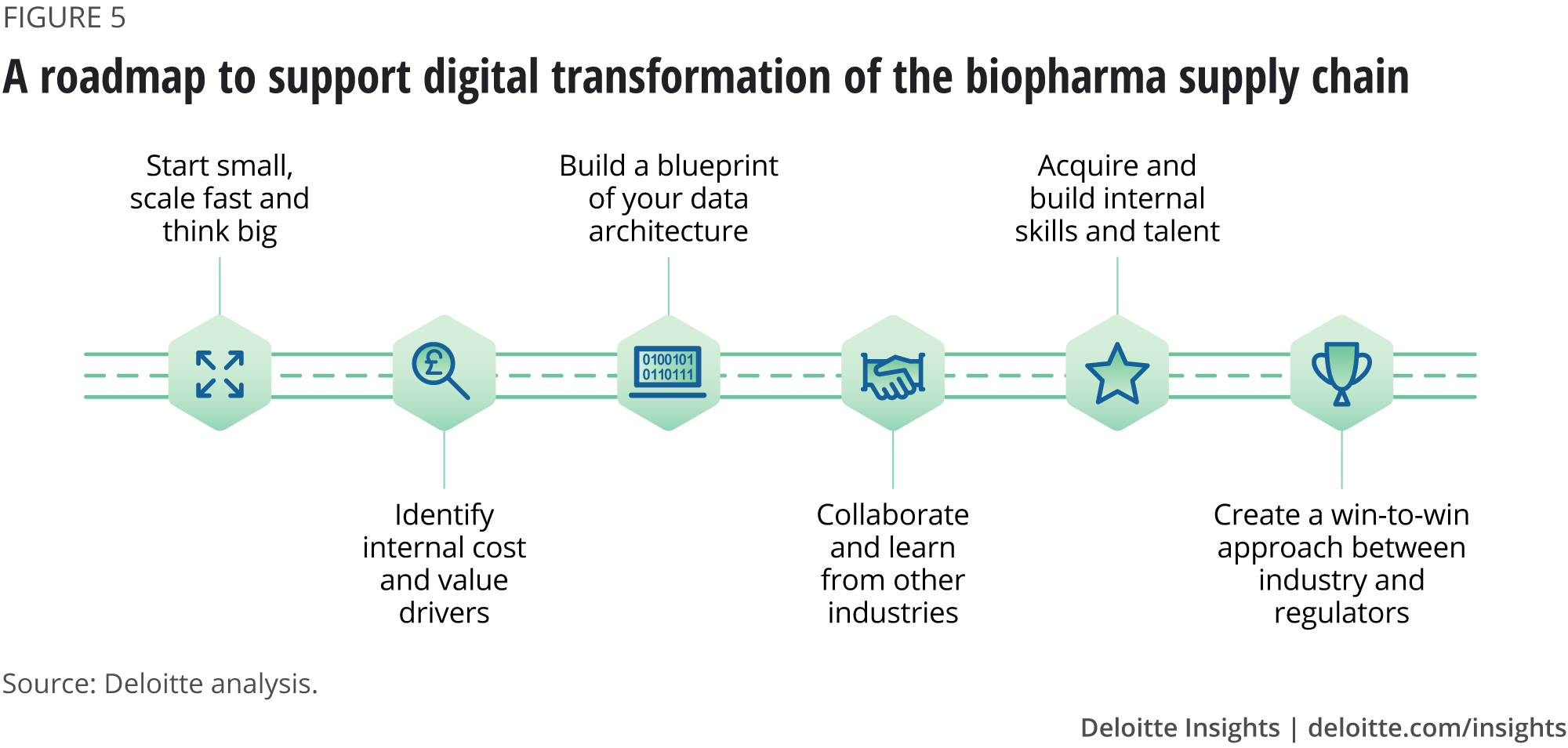
Identify internal cost and value drivers
DSNs can help biopharma deliver more value and lead to lower costs, higher efficiencies and better use of capacity across its “plan – source – make – deliver” operations. Companies with a robust knowledge of their supply chain cost drivers in each function are better positioned when deciding which AI technology pilot projects should be prioritised.
Build a blueprint of your data architecture
When transforming with AI, smart and optimised data management is essential. This is the most important consideration when planning to innovate the supply chain. Currently, inadequate IT infrastructures and a lack of interoperability standards present significant barriers to biopharma DSN implementation.18 Overcoming these hurdles can increase connectivity and begin to generate vast amounts of digital information, while ensuring data privacy. Cloud computing is a key enabler in helping to bridge the whole data ecosystem across the DSN.
Collaborate and learn from other industries
In the past three years, digital technology experts from other industries have established several collaborations focused on developing AI solutions for biopharma manufacturing. These represent a promising opportunity for biopharma companies. In addition, biopharma can also learn from other industries by acquiring their expertise and adopting their innovation models.
Acquire and build the right skills and talent
The adoption of AI and implementation of DSNs will require changes to roles and responsibilities, including employing a more diverse workforce.19 The next generation of talent will need to be agile, digitally literate and open to continuous learning. As technology and capabilities evolve, biopharma employees will need to balance the pursuit of new skills with the application of their current skill sets. For drug manufacturers that want to move into an end-to-end digital supply chain, hiring experts is a priority given the lack of dedicated teams with established knowledge on AI design thinking.
Create a win-to-win approach between industry and regulators
The regulatory environment continues to increase in complexity, and failure to comply can damage a company’s reputation and have important legal and financial consequences. In addition, biopharma companies see their own regulatory functions as a strategic asset and are streamlining systems and clinical, quality and regulatory processes to eliminate functional siloes and improve compliance efficiency. The adoption of automation and data management is also supporting biopharma in developing skills to enable them to collaborate more effectively with regulators to ensure compliance.20
The future of AI-enabled supply chains
We believe the life sciences sector is ripe for adoption of AI. The industry needs to move from just producing large amounts of data to generating actionable insights from that data. Biopharma companies should embrace advanced digital solutions and unlock their potential if they want to meet future market demands for more precise, personalised therapeutics. The expectation is that shifting from a traditional linear supply chain to an AI-enabled interconnected DSN, together with radically interoperable data, will help companies thrive. We also expect that, in response to the COVID-19 pandemic, the digital transformation of the supply chain will accelerate at an unparalleled rate and scale.
Read the full report, Intelligent drug supply chain: Creating value from AI, for more insights and get access to eight case studies.
© 2021. See Terms of Use for more information.
Explore more on Biopharma
-
Cell and gene therapies Article5 years ago
-
Winning in the cell and gene therapies market in China Article4 years ago
-
Key factors to improve drug launches Article5 years ago
-
Striving to become more patient-centric in life sciences Article5 years ago