
A new view on market access and reimbursement Launching innovative biopharma in China
7 minute read
16 March 2019
Expanding your perspective of China’s drug marketplace can open up myriad opportunities. This first in a series of four articles explores innovative alternatives in the public and self-pay markets.
In China innovative medicines have historically faced an enormous struggle to achieve reimbursement: to be included on a list of drugs for which the government will pay some of the cost.1 Any product seen as too new or too costly would likely not make it onto the list. Moreover, the list was rarely updated, meaning a lag in evaluating and accepting new medicines. But vast changes are happening in the Chinese marketplace, making it timely for biopharma companies to rethink market access and reimbursement when it comes to launching innovative medicines there; new pricing options, collaboration and creative thinking can boost your chances of success.
Shifting paradigm of reimbursement
Learn More
Explore the Launching innovative biopharma in China series
View the Life Sciences collection
Subscribe to receive more information about innovative biopharma in China
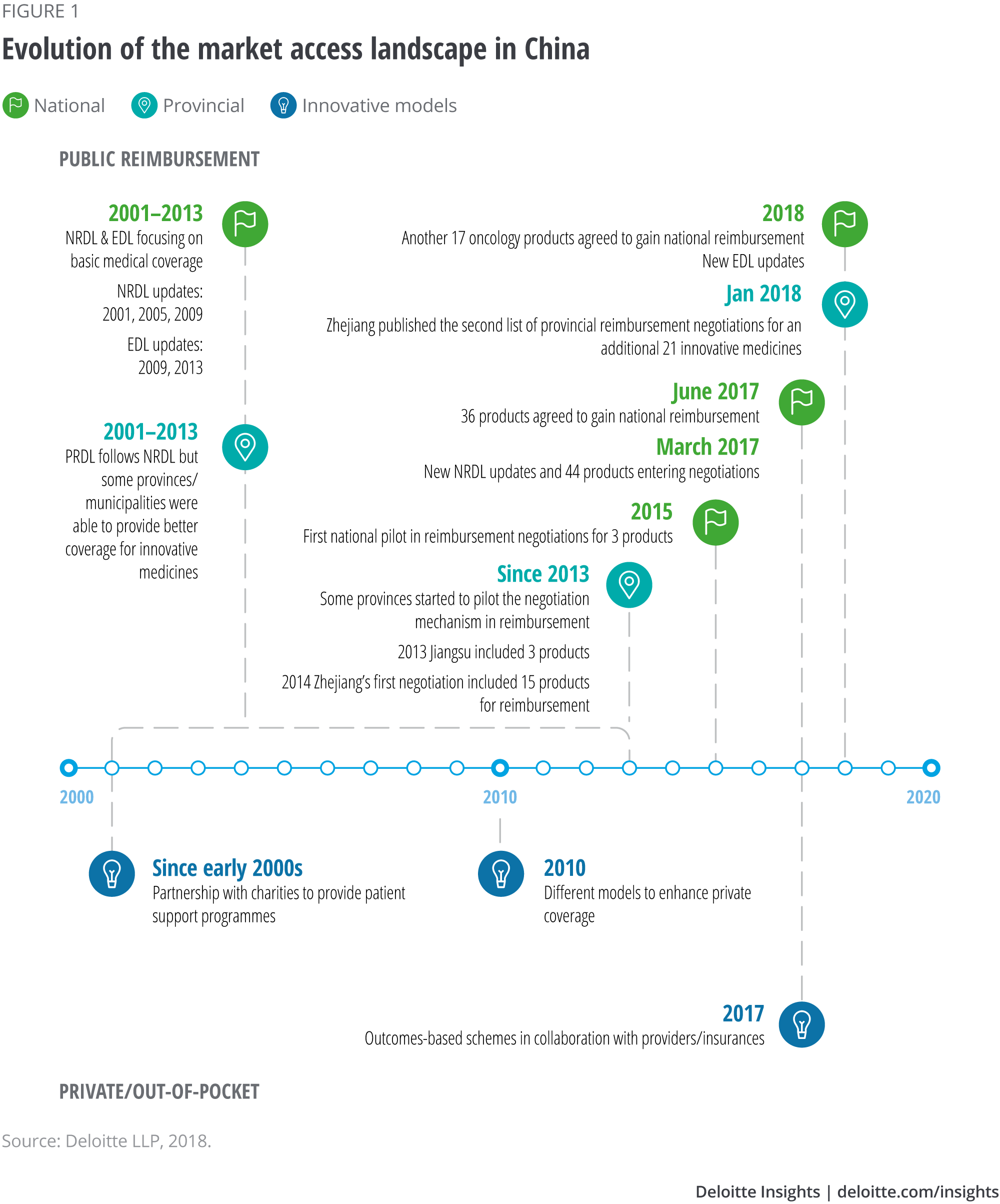
At the national level, the national reimbursement drug list (NRDL) and the essential drug list (EDL) aim to provide basic medical coverage to China’s population of 1.4 billion, typically covering well-established and relatively cheap medicines.2 Although some provinces have been able to cover additional products, overall coverage for innovative medicines has been very limited.3 Moreover, updates to the NRDL have often been significantly delayed (see Figure 1), although they should have occurred every two years – a frequency initially set by the Ministry of Health (MoH) in the early 2000s.4
After many years of delay, China updated its NRDL in early 2017 – growing the ‘Western medicine’ list by 11 per cent, to 1,297. More importantly, in addition to the updated list, a ‘to-be-negotiated’ list was published, inviting 45 medicines into price negotiations.5
Negotiable prices paving the way
The price negotiation mechanism was formally introduced at the national level for the first time in 2017, following a pilot in 2015 (see Figure 1). After a few months of negotiations, price discounts were agreed with the MoH for 36 medicines, enabling them to join the NRDL.6 The additions included expensive monoclonal antibodies drugs, established blockbuster cancer therapies and also relatively new medicines approved by the China Food and Drug Ad- ministration (CFDA). In late 2018 another 17 cancer drugs went through the same process and agreed on discounts to gain national reimbursement. Ten of those 17 drugs are medicines approved after 2017.7
China’s provinces observed the NRDL by including the products in their provincial reimbursement drug lists (PRDLs). The copayment amount for the drugs varied among provinces, meaning that patients had to pay some costs, depending on the regime adopted by each province.
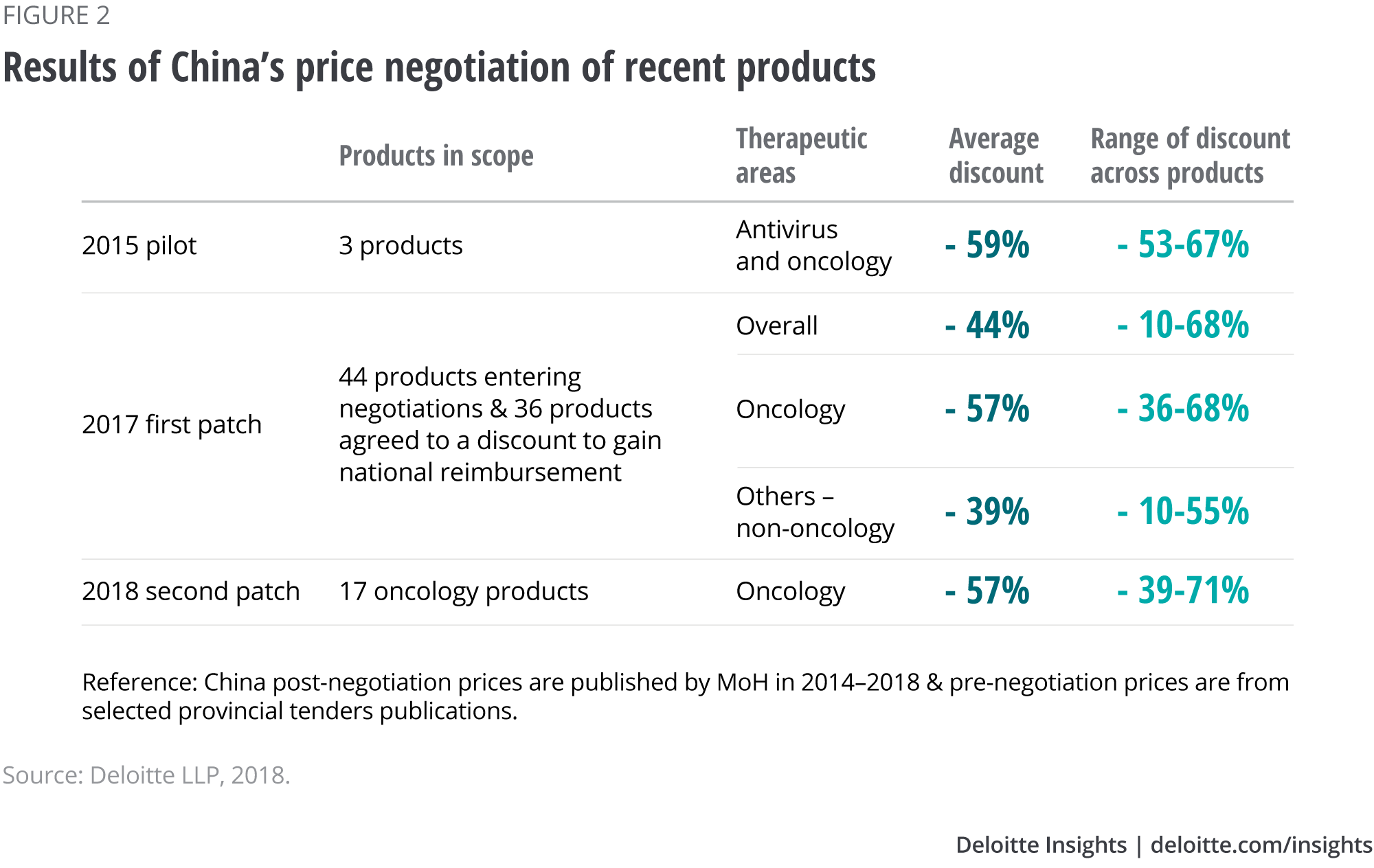
Clearly the new pricing mechanism is an encouraging sign for domestic and multinational manufacturers of innovative medicines. But reimbursement has a drawback: an average price cut of 44 per cent across 36 products in 2017, and 57 per cent in 2018.8 Oncology products, in particular, seem to experience greater price cuts than products in other therapeutic areas, possibly due to their high cost (see Figure 2).
Provincial nuances in reimbursement
Historically, provinces in China have been able to provide better coverage for innovative medicines, de- pending on affordability and willingness to pay. They have also been more proactive in experimenting and piloting different programmes to enhance patient access (see Figure 1). Some provinces introduced the price negotiation mechanism almost three years before the national authority pilot scheme in 2015.
Certain provinces will likely continue to lead the way and introduce innovative access schemes. Zhejiang is a pioneer to watch: In early 2018, this province added another 21 drugs through the negotiation process at the provincial level. The new list includes seven drugs that did not reach an agreement in the last round of provincial negotiations, in 2014, as well as some innovative medicines newly approved by the CFDA.9
Innovative programmes in the self-pay market
Although innovative products’ inclusion in the re- imbursement system has risen significantly in China during the past 12 months, a large market remains self-pay, meaning private or out-of-pocket – patients bear the costs. This has led to many innovative access programmes in the nonpublic reimbursement space, to try to improve patient affordability (see Figure 1).
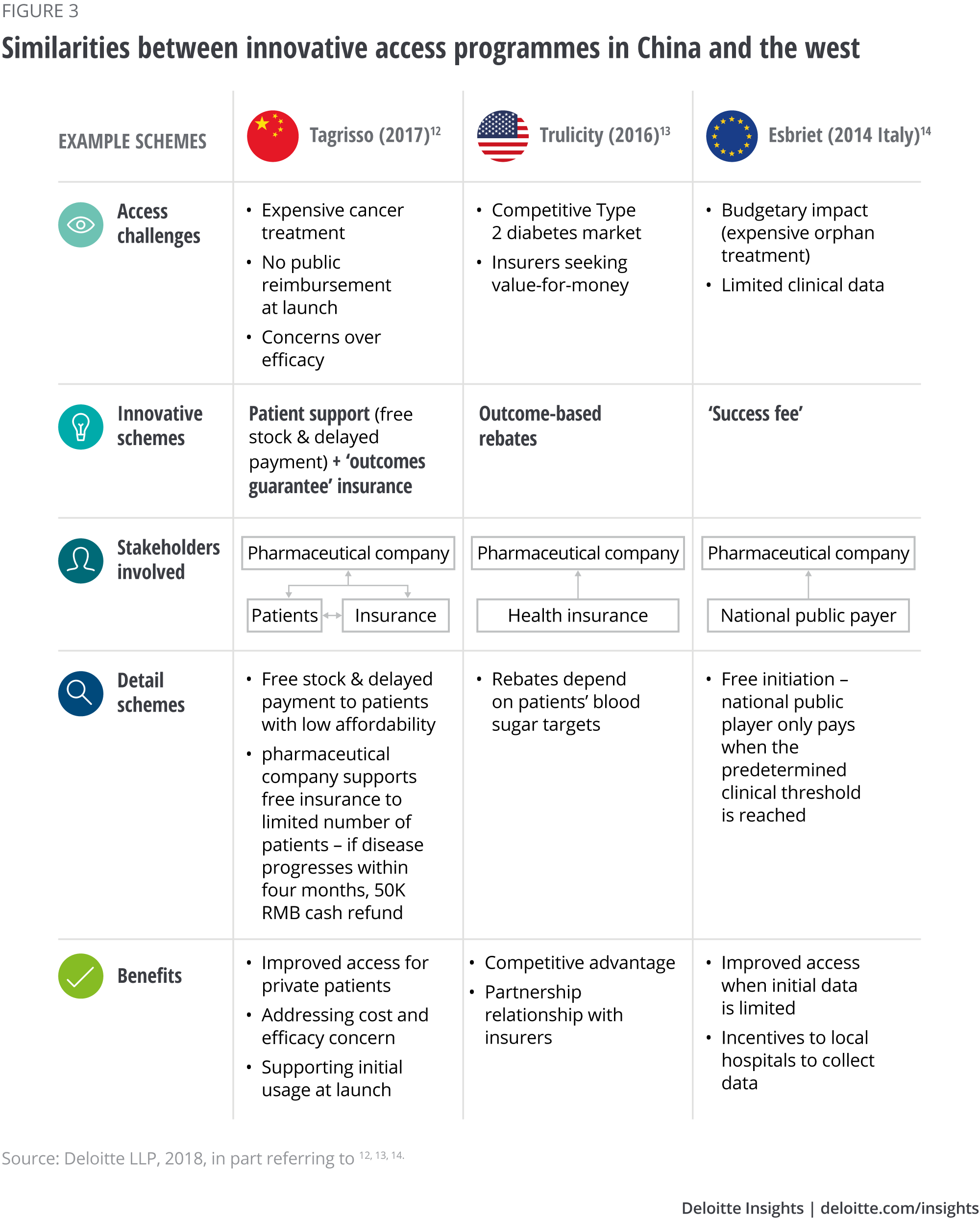
Various pharmaceutical companies participate in programmes to improve patient affordability and access to drugs; a large multinational health care company has set a precedent by heavily investing in a Shanghai innovation centre, which is due to be inaugurated in 2019.10 Moreover, starting from 2014, various manufacturers in China have tried outcome-based schemes (programmes).11 Although they focus only on the self-pay market, Deloitte’s analysis (see Figure 3) shows they share some similarities to their western counterparts: a movement toward value-based care with multiple stakeholders involved to improve patient access. Given the fast-changing health care and reimbursement landscape, it is not unreasonable to believe that outcome-based schemes with public payers may be on the horizon in China.
New perspectives for product launch teams
In Deloitte’s view, biopharma companies and global manufacturers of biological and chemical medicines would benefit from tailored launch campaigns to deftly handle the vastly changing Chinese marketplace. There are three aspects to consider in developing your strategy:
1. PURSUIT OF PUBLIC REIMBURSEMENT
Management and sales teams are encouraged not to assume that public reimbursement is impossible to obtain. The new price negotiation mechanism (at both the national and provincial levels) now offers possibilities to explore in terms of the drug’s price and the volumes that can be sold. Allocate internal investment to thoroughly research the options, and to evaluate the brand opportunity in a more sophisticated way than you would for a traditional volume-price assessment in a self-pay market. Practise robust scenario planning for forecast and launch activity. Finally, take a tactical approach to timing – considering, for example, that Zhejiang has updated its PRDL three times in the past three years,15 and that previously the national list was not updated from 2009 to 2016.
2. VIABILITY OF THE PUBLIC PAYER OPTION
Although the new reimbursement option is ex- citing news for the industry, it goes without saying that the public route may not be the best solution for all brands or companies. From the public payer perspective, various products differ in their reimbursement potential. For example, products with a differentiated clinical profile, large volume uptake and broad indication base tend to be favoured for inclusion. By contrast, niche-consumer–focused drugs or drugs with low-cost alternatives are much less appealing for public reimbursement.
Biopharma companies should get inside the heads of public payers. What are their immediate priorities? How do they perceive the product in terms of value? Conduct a holistic assessment of product characteristics to ensure effective engagement with payers. Then evaluate the profitability of commercial business cases carefully. In the past, there have been clear winners who exchanged price for volume, but also less successful cases where reimbursement did not help a brand achieve the performance expected.
3. CHOOSING THE MODEL, SHAPING THE MARKET
The landscape of both the private and public markets will keep evolving in China. Companies that want to succeed in launching future products must stay on top of the changing dynamics and continue to explore new access options for patients. Some innovative contracting programmes in the western market have not yet been widely applied in China, such as orphan disease-specific reimbursement, risk-sharing programmes or volume-based contracting, to name just a few. Those programmes make possible a win-win situation for customers and biopharma companies, if tailored to the Chinese market.
For the public market, as the introduction of price negotiation has shown, many new mechanisms began at the provincial and local levels, where some stakeholders may be more open to discuss innovative programmes. In the private sector, the evolving network of health care providers and private health insurance providers offers new opportunities to enhance patient access through different models of partnership and collaboration. Consider the different needs of customers/patients, and structure your offering to provide value to all the various stakeholders, aiming to shape the development of the market access system together.
Conclusion
Capitalising on opportunity in China requires a fresh and adaptable view of the evolving system. Beyond that, strong collaboration and organisational capabilities are a necessity – for example, in relation to government affairs and market access functions. Early on, define your product’s opportunities and obstacles, and use these to shape your launch model. This approach can lead beyond launch success to optimising the market access system as a whole. Explore further insights into launching innovative biopharmaceuticals in China with three other articles in this series, on: digital technologies, regulations and the changing health care environment.
Explore further insights into launching innovative biopharmaceuticals in China with three other articles in this series, on: digital technologies, regulations and the changing health care environment.
Read other articles in the series
-
Gain the edge in a fast-moving market Article5 years ago
-
A new view on China’s digital health care Article5 years ago









