Chemicals of Concern Risk Mitigation and Remediation has been saved

Close

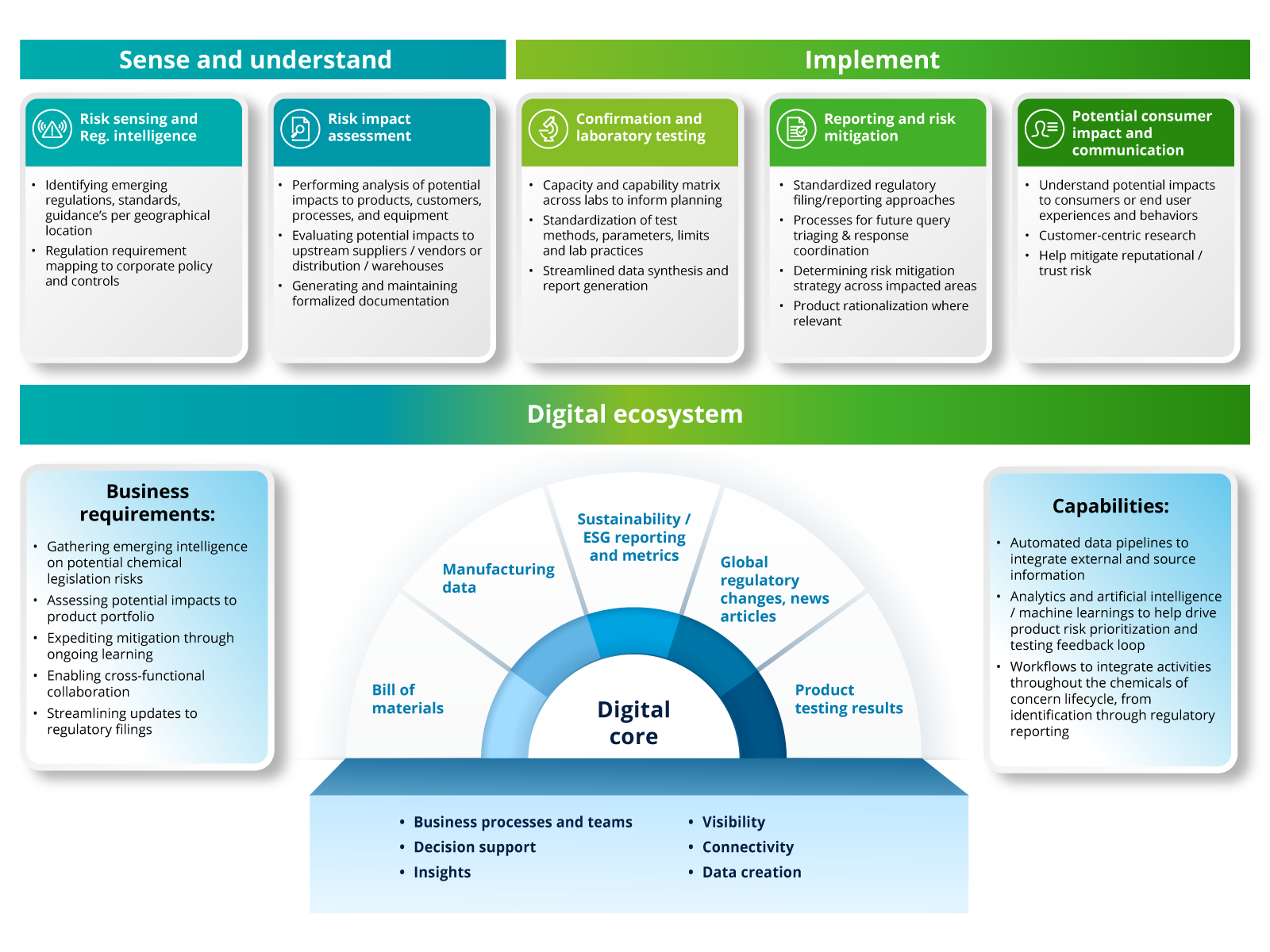
Chemicals of Concern: Evolving regulations and their impact to portfolios
In this LinkedIn Live event, Deloitte professionals discuss the proactive steps companies can take to help identify and remediate chemicals of concern throughout their portfolio and supply chain.
false