Interoperability, wellness, empowered consumers will drive the future of health has been saved

Perspectives
Interoperability, wellness, empowered consumers will drive the future of health
Health Care Current | February 12, 2019
This weekly series explores breaking news and developments in the US health care industry, examines key issues facing life sciences and health care companies, and provides updates and insights on policy, regulatory, and legislative changes.
My Take
Interoperability, wellness, empowered consumers will drive the future of health
By Steve Burrill, vice chairman, US health care leader, Deloitte LLP
Several of my Deloitte colleagues have escaped the recent sub-zero wind chills and are now in balmy Orlando for the annual HIMSS Global Conference & Exhibition. During sessions and panel discussions, they will lay out Deloitte’s vision for the future of health, which is our theme for this year’s conference. My colleagues will explain how big data can be combined with electronic health records (EHRs) to improve care quality and reduce the administrative burdens on clinicians. They will discuss the evolving partnerships between health plans and hospital systems, and they will explore ways real-world data could be used to keep people healthy and improve population health. All of these topics align with the transformation we are starting to see in health care.
The future of health that we envision is only about 20 years off, but health in 2040 will likely be a world away from what we have now. Based on emerging technology, we can be reasonably certain that digital transformation—enabled by radically interoperable data, artificial intelligence (AI), and open, secure platforms—will drive much of this change. Today’s siloed health care sectors will work more closely together to keep populations healthy, and empowered consumers will help to push this vision forward. Here’s a look at how we expect these forces will play out over the next 20 years:
- Radically interoperable data: Care coordination will become a central feature of new payment arrangements, according to Beyond the EHR—a new report from the Deloitte Center for Health Solutions that looks at technology-investment trends among hospitals. While 96 percent of hospitals have adopted electronic health records (EHRs), the shift to value-based payments will likely require integration across multiple platforms, according to the report. Google, for example, has created a cloud-based solution to tackle interoperability, which is often seen as one of the biggest challenges in health care.1 The technology will allow health care companies to manage data and perform analytics and machine learning using cloud technology.
- Wellness: By 2040 (and perhaps beginning significantly before), streams of health data—from a variety of sources—will merge to create a multi-faceted and highly personalized picture of every consumer’s wellbeing. Many of us already have wearable devices that track our steps, activity, and even our heart rates. In the future, data-gathering sensors will be so much a part of our lives that we probably won’t even notice them. Along with tracking our activity, devices of the future might also evaluate the impact the environment has on our health. While we will continue to rely on hospitals and clinicians, they will devote more of their time to keeping populations healthy. As a result, they won’t spend as much time on treating illness. Providers will need to find ways to decrease delivery costs to maintain margins. Strategies might include more remote and virtual health solutions, digitization, and advanced population management. Health plans will move beyond claims processing to focus on the wellbeing of members, according to our new report on the health plan of the future. We expect health plans will become data conveners, science and insight engines, and/or data and platform infrastructure builders. Health plans could become localized health hubs enabling the delivery of consumer-centric care models.
- Empowered consumers: Virtual interactions with patients rose from 56 percent of total interactions in 2015 to 59 percent in 2017, according to Beyond the EHR. This illustrates how we are transitioning from a business-to-business health care model to a consumer-to-business model. By 2040, the consumer—rather than health plans or clinicians—will determine when, where, and with whom care or wellness services are performed. Consumers will have access to data that details their physical, social, mental, emotional, and spiritual health. This information might be combined with other data sets and shared with physicians, health organizations, researchers, or product developers. But consumers will control how data are shared.
- Closer coordination between health plans and providers: Last September, we surveyed health care professionals from 300 primary and specialty care practices to find how receptive they were to working more closely with health plans. We identified five areas (i.e., care coordination, cost transparency, chronic care management, wellness and prevention, and practice performance) where health plans have capabilities or useful data. Many health care practitioners said they are receptive to solutions from health plans that can help improve patient experience and outcomes. Solutions that address unmet needs, complement existing capabilities, and provide insights that physician practices cannot get elsewhere could be well-received. We’ll be publishing that report later this week, so make sure you’re subscribed to receive our Deloitte health care content to read the full findings.
Along with enjoying some warm weather, I hope everyone who attends this year’s HIMSS conference will have a chance to hear Deloitte’s perspective on the future of health. By 2040, we envision an integrated system that keeps populations healthy and out of the hospital or doctor’s office. Radically interoperable data, empowered consumers, and greater coordination between all health care stakeholders could help us achieve this vision.

1 Gregory J. Moore MD, Ph.D., Google Cloud for Healthcare: new APIs, customers, partners and security updates, March 5, 2018.
Subscribe
Subscribe to receive the Health Care Current via email
In the News
HHS issues proposed rule to improve interoperability, increase patient access
On February 11, the US Department of Health and Human Services (HHS) Office of the National Coordinator for Health IT (ONC) released a proposed rule to support the secure access and exchange of electronic health information (EHI). According to HHS, the proposed rule will provide patients and physicians with tools to improve EHI access, which the agency says will encourage competition, allow for new business models in the health care market, and increase patient choice. Specifically, the proposed rule would mandate that patients can access their EHI at no cost. The proposed rule also calls for adoption of standardized application programming interfaces (APIs), which could allow patients to access their EHI via smartphone applications. Additionally, the proposed rule would implement provisions of the 21st Century Cures Act that prevent—and penalize—information blocking by outlining seven proposed exceptions to the definition of “information blocking,” including practices that prevent patient harm and assure EHI security. ONC also released a request for information for including price information within the scope of EHI.
(Source: HHS, HHS Proposes New Rules to Improve the Interoperability of Electronic Health Information, February 11, 2019)
Legislative roundup: Reintroduced bills address drug pricing, generic and biosimilar competition
- On February 5, House and Senate lawmakers reintroduced the Creating and Restoring Equal Access to Equivalent Samples (CREATES) Act, a bill that aims to increase generic-drug and biosimilar competition. Senators Chuck Grassley (R-Iowa) and Patrick Leahy (D-Vt.) reintroduced the bill, which has a bipartisan group of 29 co-sponsors. Provisions include:
- Preventing denial of drug samples to generic companies to use for testing.
- Allowing biosimilar manufacturers and generic drug makers to sue brand-name manufacturers in federal court for an injunction to receive samples for testing.
- Preventing brand-name manufacturers from using safety protocols to block access to samples.
The earlier legislation, which the pharmaceutical industry opposed, had bipartisan support, but failed to pass last year. The Association for Accessible Medicines (AAM) has expressed support for CREATES, and the Pharmaceutical Research and Manufacturers of America (PhRMA) agreed that efforts to block competition by improperly invoking safety protocols should be taken seriously. The trade group intends to work with lawmakers to further improve the bill. The CREATES Act also received letters of support from 50 other stakeholder associations and advocacy groups.
- On February 7, Congressman Doug Collins (R-Ga.) revived two other bills intended to address drug costs. The bills, which have bipartisan support, address the interactions between pharmacies and pharmacy benefit managers (PBMs). One of the two bills, the Phair Pricing Act, would require:
- Applying price concessions between pharmacies and PBMs to the point of sale in Medicare Advantage (MA), Part D, and the Federal Employees Health Benefit Program.
- PBMs to disclose any concessions they receive to the US Centers for Medicare and Medicaid Services (CMS) to help the agency align market incentives and gain transparency on drug costs.
- Under the second bill, called the Prescription Drug Price Transparency Act:
- PBMs would have to update their maximum allowable costs lists, which set the maximum reimbursement rates given to pharmacies for drugs, to prevent delays in price updates.
- PBMs would have to disclose the information used to determine maximum allowable cost (MAC) price determinations.
- PBMs would not be allowed to limit the pharmacies that Medicare beneficiaries and federal employees use to obtain medications.
The legislation follows a recent proposed rule from HHS that would levy fines for drug rebates under the Anti-Kickback Statute. The proposed rule was criticized by the Pharmaceutical Care Management Association (PCMA), but received support from PhRMA, which applauded the proposed rule’s potential to help patients with chronic diseases benefit from rebates and discounts (see the February 5, 2019 Health Care Current).
Facility fees, hospital prices drive health care inflation, study finds
Hospital prices are the main driver of health care inflation, according to a study published in Health Affairs on February 4. The study analyzed claims data from the Health Care Cost Institute (HCCI) from people who had employer-sponsored health coverage between 2007 and 2014. Researchers also examined data from hospitals and from a market intelligence database.
The data showed growth in physician and hospital prices for inpatient care, outpatient care, and four high-volume services, including knee-replacement surgery. A January study from a national health insurer also noted a significant increase in costs associated with knee-replacement surgeries (see the February 5, 2019 Health Care Current).
From 2007 to 2014, the Health Affairs study found that:
- Hospital prices for inpatient care increased by 42 percent while physician prices increased by 18 percent.
- Hospital prices for outpatient care increased by 25 percent while physician prices increased by 6 percent.
- Rising facility fees were the greatest contributor to the growth in total cost for care, and the spike in fees increased by 97 percent for knee-replacement surgeries.
Hospital prices increased more than physician prices for both inpatient and outpatient care for the health services analyzed in the study, including knee-replacement surgeries, as illustrated below:
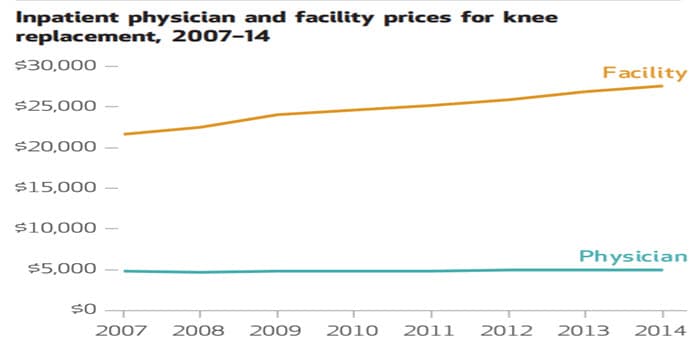
(Source: Health Affairs, Hospital Prices Grew Substantially Faster Than Physician Prices For Hospital-Based Care In 2007–14, February 4, 2019)
Insurers appeal to Supreme Court in effort to recover billions in risk-corridor payments
Several health plans are asking the US Supreme Court to consider claims that they are owed billions of dollars from the government under the Affordable Care Act’s (ACA) risk-corridors program. The health plans say the federal government did not make required payments after Congress—in late 2014—passed an appropriations rider that required the program to be budget neutral. Making the program budget neutral resulted in a $12 billion shortfall to the government. In June 2018, a federal appellate court rejected the claims, leaving the insurers to appeal the ruling to a higher court.
Qualified health plans in the individual and small-group markets participated in the temporary risk-corridors program from 2014 to 2016. The program’s primary goals included protecting health plans from adverse selection by limiting their potential losses and gains. Health plans that enrolled less-costly customers were required to pay into a common fund that would offset the costs other health plans experienced when they enrolled sicker customers. Many participating plans lost money because the money owed to health plans was much higher than the money collected (see the June 12, 2018 Health Care Current).
Health insurers appealing the earlier ruling include Blue Cross and Blue Shield of North Carolina, Moda Health, Land of Lincoln Health, and Maine Community Health Options.
State of the Union address proposes health initiatives, calls for research funding
The 2019 State of the Union address, which was delivered on February 5, proposed several health care initiatives and outlined goals to improve access to treatment, increase funding for research, and tackle rising costs. Proposals include:
- Increasing investments in HIV prevention programs, such as the Ryan White HIV/AIDS Program, and directing funding to launch new programs, through community health centers, to provide preventative medication to high-risk individuals. Data would be used to prevent the spread of HIV and provide direct funding to areas in greatest need.
- Asking Congress to allocate more that $500 million over the next 10 years to fund research for childhood cancer. Previously, the administration took steps to improve care for terminally ill patients by signing the Right to Try Act in May 2018 (see the June 5, 2018 Health Care Current).
- Continuing efforts to reduce the price of prescription drugs, as outlined in the Blueprint to Lower Drug Prices and Reduce Out-of-Pocket Costs (see the January 8, 2019 My Take). Several committees in the House and Senate have begun to hold hearings focused on assuring price transparency and reducing costs for prescription drugs (see the February 5, 2019 Health Care Current).
- Expanding efforts to reduce “surprise billing” where patients are billed for care that is much more expensive than anticipated—or is not covered by their insurance.
Senators probe insurers and hospitals on ‘surprise billing’ practices
A bipartisan group of Senators sent a letter to health plans and hospitals requesting data on surprise medical billing practices. Surprise billing is one area policymakers are targeting to reduce health care costs for consumers, and this letter is the latest effort to address high medical bills—and the rising cost of health care. Initiated by Bill Cassidy, M.D., (R-La.), the senators requested detailed information from health plans that explained how out-of-network payments compare to Medicare rates, provider charges, and in-network rates. From hospitals, the senators requested information about the percentage of specialty care billed as out-of-network at in-network hospitals.
2018 was a record year for device innovation, FDA says
The US Food and Drug Administration (FDA) approved a record 106 novel medical devices in 2018, FDA Commissioner Scott Gottlieb said in a prepared statement on January 28. This surpasses the 40-year approval record set in 2017, which was 99, Gottlieb noted. The charts below show the steady gains in FDA approvals of novel medical devices since 2013:
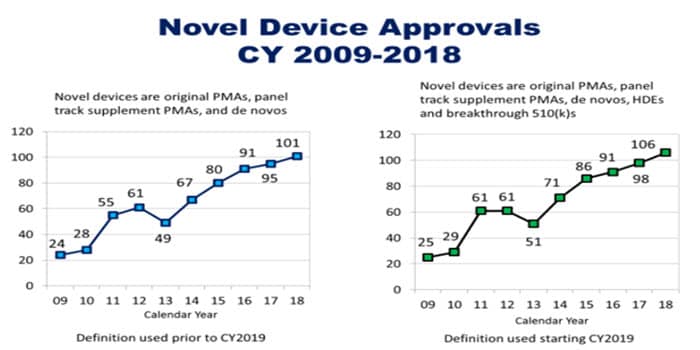
According to Gottlieb, FDA has also granted 112 Breakthrough Device Designation requests since 2015. In 2018, the agency approved or cleared nine breakthrough devices. The voluntary Breakthrough Devices Program is aimed at medical devices and products that allow for more efficient treatment of life-threatening or debilitating illnesses.
(Source: FDA, Statement from FDA Commissioner Scott Gottlieb, M.D., and Jeff Shuren, M.D., Director of the Center for Devices and Radiological Health, on a record year for device innovation, January 28, 2019)
Related: On February 4, FDA released a final guidance outlining how it will apply expanded least-burdensome review provisions to a medical device’s total product lifecycle—rather than only the “premarket” phase. This final guidance, “The Least Burdensome Provisions: Concept and Principles,” sets forth updated least-burdensome provisions from the 21st Century Cures Act and replaces a 2002 version on the same topic.
Breaking Boundaries
Japan is pursuing high-tech solutions for its aging population
At some senior living facilities in Japan, residents have ultrasonic bladder sensors that send a signal to caregivers when they need to use the restroom. The device is in use at 150 senior facilities across the country. The startup that developed the device is now exploring similar opportunities in the US. Health care spending in Japan is about 10.7 percent of its gross domestic product (GDP), according to the Organization for Economic Cooperation and Development (OECD). This is far lower than in the US, where health care accounts for 17.2 percent of GDP—but Japan is still focused on holding down costs.
Providing products and services to Japan’s millions of seniors is a national priority. One goal is to keep seniors in their homes longer—and to use digital technology to reduce the burden on caregivers. Z-Works, a Japanese IT company, is working to improve patient-monitoring systems. Some of the company’s devices can remotely monitor a patient’s breathing and heartbeat—from a hospital bed or anywhere in the facility—and stream data to nurses’ stations.
Innovation is also happening outside of the private sector. Japan’s government is trying to make it easier for startups and other technology companies to gain access to medical records to spur innovation. The government is launching a certification program, and a stakeholder panel is working on privacy and ethics rules. The government also seeks to fund studies that explore ways for digital technology to augment traditional treatments. For example, a study could look at how a wearable device could improve adherence to medication regimens by transmitting reminders to the patient.
Some US startups have launched senior-focused technology
Like their Japanese counterparts, startups in the US are also developing and launching products to help our aging population. A company called Rendever has created a virtual-reality (VR) experience for people who have trouble traveling or leaving their homes. The company transports seniors to beautiful settings in nature with the help of VR headsets and a tablet loaded up with various VR experiences. Users are seated, but are encouraged to twist and move their upper bodies. Seismic, another startup, is tackling loss of mobility through a skin-tight suit designed to boost strength in aging hips and legs. The suit uses compression and tiny motors that stimulate muscles during activities such as standing and sitting. The users still need to move themselves, but the suit makes it easier.
(Source: The Wall Street Journal, In fast-aging Japan, elder care is a high-tech pursuit, January 12, 2019)
Recommendations
Health Care Blog
A View from the Center

